Question: A solution with an ionic strength of 0.10 M containing 0.010 0 M phenylhydrazine has a pH of 8.13. Using activity coefficients correctly, find pKa
A solution with an ionic strength of 0.10 M containing 0.010 0 M phenylhydrazine has a pH of 8.13. Using activity coefficients correctly, find pKa for the phenylhydrazinium ion found in phenylhydrazine hydrochloride. Assume that λBH+ = 0.80.
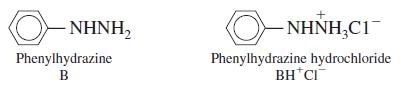
, O- NHNH,C1- Phenylhydrazine Phenylhydrazine hydrochloride BH*CI B
Step by Step Solution
3.28 Rating (169 Votes )
There are 3 Steps involved in it
The chemical reaction between phenylhydrazine B an... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
878_61d6ac3420af4_829410.pdf
180 KBs PDF File
878_61d6ac3420af4_829410.docx
120 KBs Word File


