Question: (a) Write a balanced equation for the reaction and calculate E for the reaction. (b) Predict whether an equimolar mixture of PuO 2 2 +
(a) Write a balanced equation for the reaction and calculate E° for the reaction.
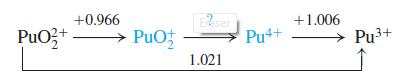
(b) Predict whether an equimolar mixture of PuO22+ and PuO+2 will oxidize H2O to O2 at a pH of 2.00 and PO2 = 0.20 bar. Will O2 be liberated at pH 7.00?
+0.966 +1.006 PuO3+ PuOf Pu4+ -> Pu3+ 1.021
Step by Step Solution
3.41 Rating (182 Votes )
There are 3 Steps involved in it
a The balanced chemical reaction is given as PuO 2 e 4H Pu 4 2H 2 O The stepwise reac... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
878_61d6ac342114b_829498.pdf
180 KBs PDF File
878_61d6ac342114b_829498.docx
120 KBs Word File


