An ammonia gas-sensing electrode gave the following calibration points when all solutions contained 1 M NaOH. A
Question:
An ammonia gas-sensing electrode gave the following calibration points when all solutions contained 1 M NaOH.
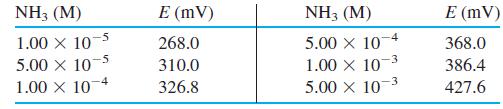
A dry food sample weighing 312.4 mg was digested by the Kjeldahl procedure (Section 10-8) to convert all nitrogen into NH4+. The digestion solution was diluted to 1.00 L, and 20.0 mL were transferred to a 100-mL volumetric flask. The 20.0-mL aliquot was treated with 10.0 mL of 10.0 M NaOH plus enough NaI to complex the Hg catalyst from the digestion and diluted to 100.0 mL. When measured with the ammonia electrode, this solution gave a reading of 339.3 mV. Calculate the wt% nitrogen in the food sample.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





