Question: Consider the following equilibria in aqueous solution: (a) Calculate the numerical value of the equilibrium constant for the reaction (b) Calculate the concentration of AgCl(aq)
Consider the following equilibria in aqueous solution:
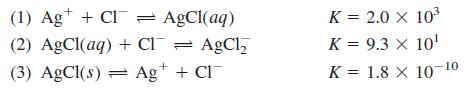
(a) Calculate the numerical value of the equilibrium constant for the reaction
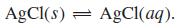
(b) Calculate the concentration of AgCl(aq) in equilibrium with excess undissolved solid AgCl.
c) Find the numerical value of K for the reaction
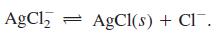
(1) Ag* + Cl = AgCl(aq) (2) AgCl(aq) + Cl = AgCl, K = 2.0 X 103 %3D K = 9.3 x 10' (3) AgCl(s) = Ag+ + Cl K = 1.8 X 10-10
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
a The overall reaction is Ag Cl Ag Cl We can write the equilibrium expression for this reaction as K ... View full answer

Get step-by-step solutions from verified subject matter experts


