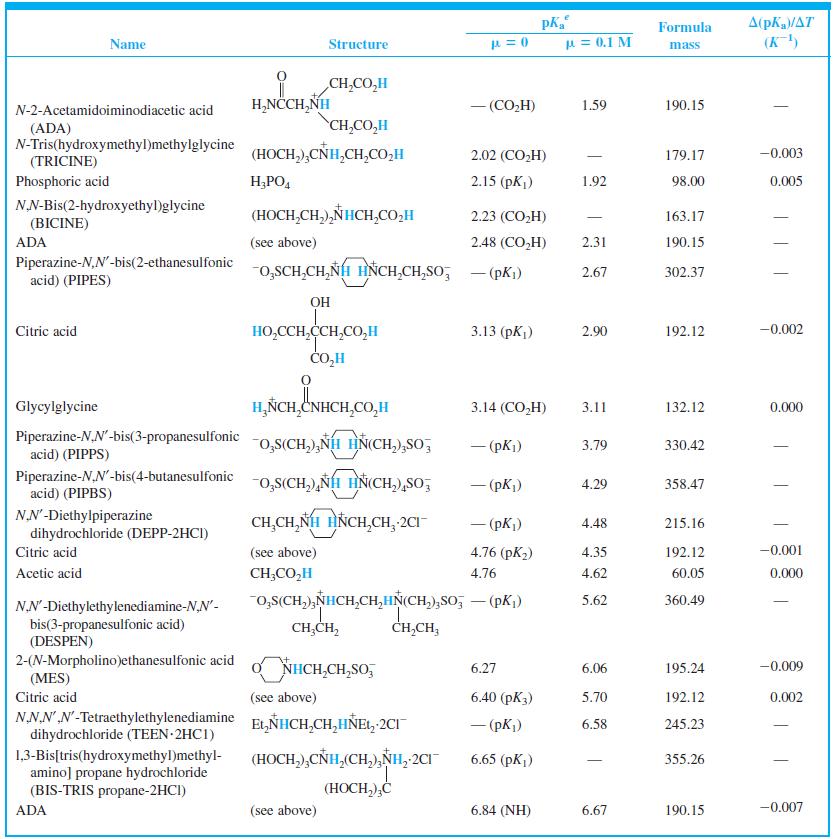
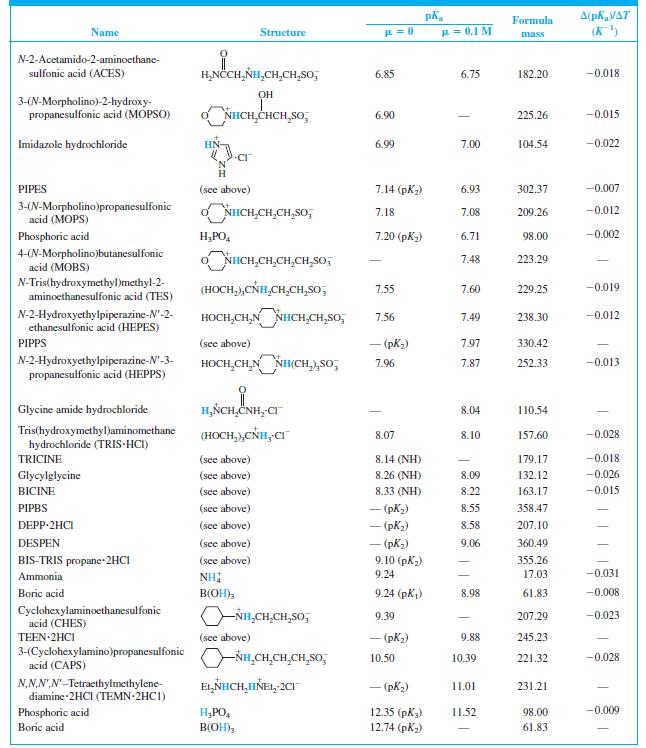
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH. Table 8-2 A(pKa/AT (K)
Question:
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH.
Table 8-2
Transcribed Image Text:
A(pKa/AT (K) Formula Name Structure u = 0.1 M mass CH,CO,H H,NCCH,NH - (CO,H) 1.59 190.15 N-2-Acetamidoiminodiacetic acid CH,CO,H (ADA) N-Tris(hydroxymethyl)methylglycine (TRICINE) (HOCH,),CÑH,CH,CO,H 2.02 (CO,H) 179.17 -0.003 Phosphoric acid H;PO, 2.15 (pK) 1.92 98.00 0.005 NN-Bis(2-hydroxyethyl)glycine (HOCH,CH,),NHCH,CO,H 2.23 (CO,H) 163.17 | (BICINE) ADA (see above) 2.48 (СO,H) 2.31 190.15 Piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES) -o,SCH,CH,NH HNCH,CH,So; - (pKi) 2.67 302.37 OH HO,CCH,CCH,CO,H CO,H Citric acid 3.13 (pKj) 2.90 192.12 -0.002 Glycylglycine H,ÑCH,CNHCH,CO,H 3.14 (CO,H) 3.11 132.12 0.000 Piperazine-N,N'-bis(3-propanesulfonic acid) (PIPPS) "o,S(CH,),NÍH HN(CH,),SO, - (pK) 3.79 330.42 Piperazine-N,N'-bis(4-butanesulfonic acid) (PIPBS) "0,S(CH,),ÑH HN(CH,),SO, 4.29 358.47 ('yd) – NN'-Diethylpiperazine dihydrochloride (DEPP-2HCI) CH,CH, NH HNCH,CH, 2CI - (pK) 4.48 215.16 Citric acid (see above) 4.76 (pK2) 4.35 192.12 -0.001 Acetic acid CH;CO,H 4.76 4.62 60.05 0.000 o,s(CH,),NHCH,CH,HN(CH,),So, – (pK,) 5.62 360.49 NN'-Diethylethylenediamine-N,N'- bis(3-propanesulfonic acid) (DESPEN) CH,CH, CH,CH, 2-(N-Morpholino)ethanesulfonic acid (MES) Citric acid NHCH,CH,SO, 6.27 6.06 195.24 -0.009 (see above) 6.40 (рК3) 5.70 192.12 0.002 NN.NN'-Tetraethylethylenediamine dihydrochloride (TEEN 2HC1) EL,NHCH,CH,HÑE1, 2CI - (pK) 6.58 245.23 (HOCH,),CNH,(CH,),NH, 2CI 1,3-Bis[tris(hydroxymethyl)methyl- amino] propane hydrochloride (BIS-TRIS propane-2HCI) 6.65 (рK,) 355.26 (HOCH,),Ć ADA (see above) 6.84 (NH) 6.67 190.15 -0.007
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
To find the equilibrium constant for the reaction of MES 2NMorpholinoethanesulfonic acid with NaOH w...View the full answer

Answered By

Rayan Gilbert
I have been teaching since I started my graduation 3 years ago. As a student, working as Teacher/PA has been tough but made me learn the needs for student and how to help them resolve their problems efficiently. I feel good to be able to help out students because I'm passionate about teaching. My motto for teaching is to convey the knowledge I have to students in a way that makes them understand it without breaking a sweat.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Find the equilibrium constant for the reaction 2NO + O2 2NO2 from the elementary reactions in Table A.10 to answer which of the nitrogen oxides, NO or NO2; is the more stable at...
-
Find the equilibrium constant for the reaction 2NO + O2 2NO2 from the elementary reactions in Table A.11 to answer which of the nitrogen oxides, NO or NO2, is the more stable at ambient conditions?...
-
The equilibrium constant for the reaction of NH3 (aq) + H2O NH+4 + OH- is Kb = 1.479 10-5 at 5C 1.570 10 -5 at 10oC (a) Assuming H and S are constant in the interval 5 - 10C (probably a good...
-
There are major responsibilities of system administrator as listed below: o Start-up and shut down the system o Performance tuning o Managing user accounts o System security o Backup and recovery o...
-
You are asked to supervise a new hire in your firm's tax department. The person has been working on the tax return of a corporation that is a new client of the firm. Help the person put together the...
-
Consider a signal x(t) = 4cos(2t) defined for < t < . For the following values of the sampling period T s generate a discretetime signal x(n) = x(nT s ) = x(t) t=nTs . (i) T s = 0.1, (ii) T s =...
-
Jim makes a deposit of $\$ 12,000$ in a bank account. The deposit is to earn interest annually at the rate of $9 \%$ percent for seven years. (a) How much will Jim have on deposit at the end of seven...
-
The cash records and bank statement for the month of July for Jim Incorporated are shown below. Additional information: a. The difference in the beginning balances in the companys records and the...
-
Sunland Manufacturing uses a job order cost system. On April 1, the company has Work in Process Inventory of $7,130 and two jobs in process: Job No. 221, $3,370, and Job No. 222, $3,760. During...
-
The Jacksons want to start investing $600 monthly to achieve their stated short- and long-term objectives. Which of the following monthly investments is most appropriate for them at this time and...
-
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH.
-
When 22.63 mL of aqueous NaOH were added to 1.214 g of cyclohexylaminoethanesulfonic acid (FM 207.29, structure in Table 8-2) dissolved in 41.37 mL of water, the pH was 9.24. Calculate the molarity...
-
Matt is self- employed as a carpenter. He made $ 42,000 after expenses in 2012. How much did he contribute to FICA taxes?
-
Melissa is a sole trader. Her capital gains and capital losses for 2021-22 are 27,000 and 700 respectively. She has capital losses brought forward from 2020-21 of 12,900 and she also has unrelieved...
-
a. Jim and Mary are about to be married. Mary is a wealthy actress. Jim is a struggling artist. Both agree that it would be a good idea to have a premarital agreement. Mary suggests that Jim make an...
-
Frank began trading on 1 July 2019. Identify the basis periods for his first four tax years if he: (a) chooses 30 June as his annual accounting date and prepares his first accounts for the year to 30...
-
Ian is a freelance television technician. In a typical tax year he works for approximately 20 separate TV companies. None of his engagements with any of these companies lasts for more than 10 days at...
-
Olive ceases trading on 31 May 2021. Her recent adjusted trading profits/(losses) are: Overlap relief of 7,140 is available. Calculate the terminal loss and show how this would be relieved, assuming...
-
Brad Jackson works for a Bob's Burgers takeout for straight-time earnings of $10.50 per hour with time and a half for hours in excess of 35 per week. Jackson's payroll deductions include income tax...
-
Teasdale Inc. manufactures and sells commercial and residential security equipment. The comparative unclassified balance sheets for December 31, 2015 and 2014 are provided below. Selected missing...
-
For the system in Problem P6.6, suppose your design requirement was to minimize the force necessary to hold the gate closed. Would you rather put the hinge at the top of the gate or at the bottom?...
-
Make an order-of-magnitude estimate for a safety floatation device that can be used by children who are playing in a swimming pool. The design concept is for one inflatable, annular, plastic balloon...
-
An ancient kings supposedly golden crown had a mass of 3 kg, but it was actually made by a dishonest metal smith from an equal mix of gold (1.93 10 4 kg/m 3 ) and silver (1.06 10 4 kg/m 3 ). (a)...
-
Estimate the final temperature in Celsius of the combination if I drop 5 rolls of pennies at 2 0 C ( each roll is 5 0 pennies stacked together; assume they are pure copper ) into a cup of recently -...
-
A 100 gram mass is hung of then end of a cantilever beam . The beam is made of brass, and is 30 cm long (), 2 cm wide, and 3 mm thick. Find the resulting deflection ()
-
The nitrogen-vacancy (NV) and silicon-vacancy (SiV) defects in diamond can lead to many exciting quantum nanotechnologies in physical and biological sciences, including single-photon sensors,...

Study smarter with the SolutionInn App


