When 22.63 mL of aqueous NaOH were added to 1.214 g of cyclohexylaminoethanesulfonic acid (FM 207.29, structure
Question:
When 22.63 mL of aqueous NaOH were added to 1.214 g of cyclohexylaminoethanesulfonic acid (FM 207.29, structure in Table 8-2) dissolved in 41.37 mL of water, the pH was 9.24. Calculate the molarity of the NaOH.
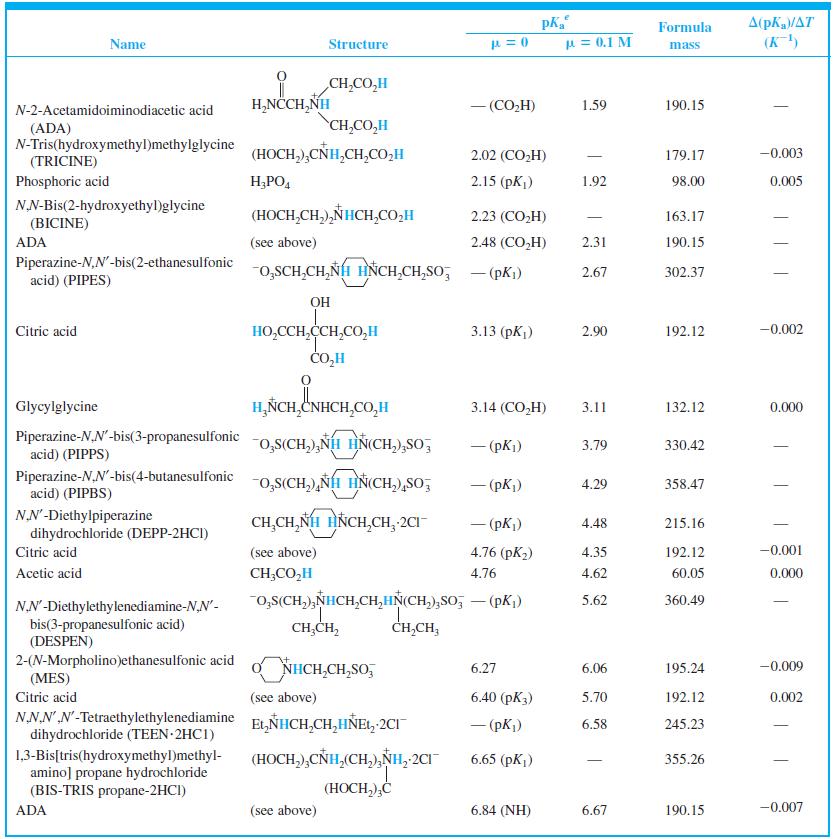
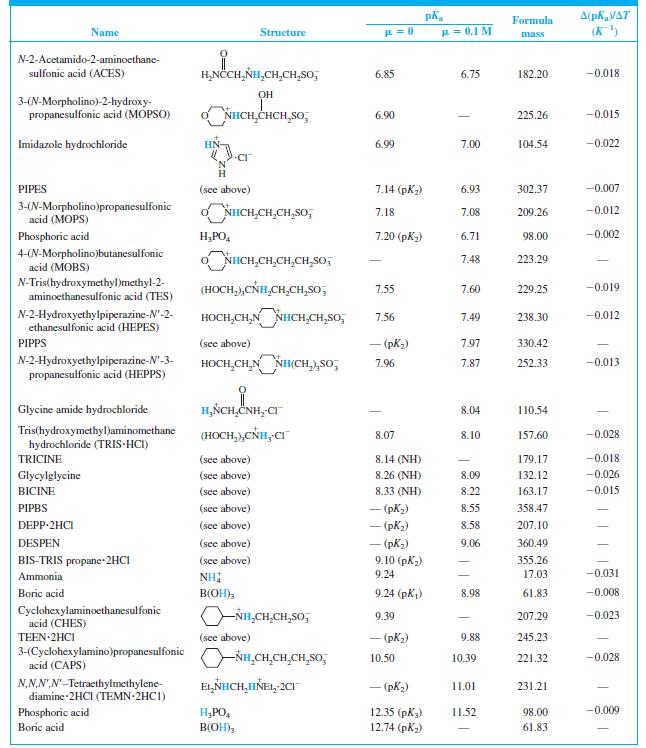
Table 8-2
Transcribed Image Text:
A(pKa/AT (K) Formula Name Structure u = 0.1 M mass CH,CO,H H,NCCH,NH - (CO,H) 1.59 190.15 N-2-Acetamidoiminodiacetic acid CH,CO,H (ADA) N-Tris(hydroxymethyl)methylglycine (TRICINE) (HOCH,),CÑH,CH,CO,H 2.02 (CO,H) 179.17 -0.003 Phosphoric acid H;PO, 2.15 (pK) 1.92 98.00 0.005 NN-Bis(2-hydroxyethyl)glycine (HOCH,CH,),NHCH,CO,H 2.23 (CO,H) 163.17 | (BICINE) ADA (see above) 2.48 (СO,H) 2.31 190.15 Piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES) -o,SCH,CH,NH HNCH,CH,So; - (pKi) 2.67 302.37 OH HO,CCH,CCH,CO,H CO,H Citric acid 3.13 (pKj) 2.90 192.12 -0.002 Glycylglycine H,ÑCH,CNHCH,CO,H 3.14 (CO,H) 3.11 132.12 0.000 Piperazine-N,N'-bis(3-propanesulfonic acid) (PIPPS) "o,S(CH,),NÍH HN(CH,),SO, - (pK) 3.79 330.42 Piperazine-N,N'-bis(4-butanesulfonic acid) (PIPBS) "0,S(CH,),ÑH HN(CH,),SO, 4.29 358.47 ('yd) – NN'-Diethylpiperazine dihydrochloride (DEPP-2HCI) CH,CH, NH HNCH,CH, 2CI - (pK) 4.48 215.16 Citric acid (see above) 4.76 (pK2) 4.35 192.12 -0.001 Acetic acid CH;CO,H 4.76 4.62 60.05 0.000 o,s(CH,),NHCH,CH,HN(CH,),So, – (pK,) 5.62 360.49 NN'-Diethylethylenediamine-N,N'- bis(3-propanesulfonic acid) (DESPEN) CH,CH, CH,CH, 2-(N-Morpholino)ethanesulfonic acid (MES) Citric acid NHCH,CH,SO, 6.27 6.06 195.24 -0.009 (see above) 6.40 (рК3) 5.70 192.12 0.002 NN.NN'-Tetraethylethylenediamine dihydrochloride (TEEN 2HC1) EL,NHCH,CH,HÑE1, 2CI - (pK) 6.58 245.23 (HOCH,),CNH,(CH,),NH, 2CI 1,3-Bis[tris(hydroxymethyl)methyl- amino] propane hydrochloride (BIS-TRIS propane-2HCI) 6.65 (рK,) 355.26 (HOCH,),Ć ADA (see above) 6.84 (NH) 6.67 190.15 -0.007
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
So the reaction between acid and base is 11 mole ratio Volume of NaOH solution V 1 2263mL concent...View the full answer

Answered By

Parvathy D nair
Hi..it s me PARVATHY ..hails from KERALA ..i am a professional tutor and working in online platform.i have a experience of 2 years and solved more than 10000 answers through online platforms.i am a professional anchor also.i am a degree holder and doing bsc in zoology.currently waiting for MBBS admission.teaching is my passion.it s not only a job for me,,it s a devine profession.i see all my Students as my little brother and sisters..
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
When 5.00 mL of 0.1032 M NaOH were added to 0.1123 g of alanine (FM 89.093) in 100.0 mL of 0.10 M KNO3, the measured pH was 9.57. Use activity coefficients to find pK2 for alanine. Consider the ionic...
-
When 100.0 mL of a weak acid were titrated with 0.093 81 M NaOH, 27.63 mL were required to reach the equivalence point. The pH at the equivalence point was 10.99. What was the pH when only 19.47 mL...
-
When an ?-hydroxy amide is treated with Br2 in aqueous NaOH under Hofmann rearrangement conditions, loss of CO2 occurs and a chain-shortened aldehyde is formed. Propose a mechanism. NH2 NH3 Br2...
-
Carlberg Company has two manufacturing departments, Assembly and Painting. The Assembly department started 11,000 units during November. The following production activity in both units and costs...
-
A taxpayer works for a law firm. You are working on her tax return. She tells you that she does not have any receipts for her daily parking because the firm pays the parking lot directly for an...
-
What is the enterprise data warehouse (EDW) architecture?
-
Suppose you make 15 equal annual deposits of \($1,000\) each into a bank account paying 5% interest per year. The first deposit will be made one year from today. How much money can be withdrawn from...
-
Abbey Park was organized on April 1, 2016, by Trudy Crawford. Trudy is a good manager but a poor accountant. From the trial balance prepared by a part-time bookkeeper Trudy prepared the following...
-
On January 1, 2024, Kroll Corporation paid $2,559,000 for 31 percent of the outstanding voting stock of Sharon, Incorporated, and appropriately applied the equity method for its investment. Any...
-
The Fashion Rack has a monthly accounting period. All transactions are recorded in a general journal. Postings are made from the general journal to the accounts receivable ledger, accounts payable...
-
Find the equilibrium constant for the reaction of MES (Table 8-2) with NaOH. Table 8-2 A(pKa/AT (K) Formula Name Structure u = 0.1 M mass CH,CO,H H,NCCH,NH - (CO,H) 1.59 190.15...
-
Why is the equivalence-point pH necessarily below 7 when a weak base is titrated with strong acid?
-
Household overall expenses over last 12 months (EXPENSHILO: 1 = Unusually high, 2 = Unusually low, 3 = Normal). Examine whether there are differences between the middle-class men and women with...
-
What are the requirements for entering a valid premarital agreement in your state?
-
Closely examine each of the following situations. Determine which restraints on marriage, if any, are enforceable. Give specific reasons you think the restraint is or is not enforceable. a. John is...
-
Jim is the managing director of a company and earns a basic salary in 2021-22 of 225,000. He receives benefits from the company during the year as follows: (a) He is provided with the use of a...
-
In 2021-22, Ahmed has capital gains of 130,000 and allowable losses of 24,000. He also has capital losses brought forward of 3,700. Ahmed's taxable income for 2021-22 (after deduction of the personal...
-
Tom and Mary execute a separation agreement on February 17, 1998, in which they mutually release all rights (dower, curtesy, election, etc.) in each others estate. They ceased living together on...
-
Sylvia Chan is a clerk in the shoe department of the Hudson's Bay store in Winnipeg. She earns a base monthly salary of $1,875 plus a 7 percent commission on her sales. Through payroll deductions,...
-
Global.asax is used for: a. declare application variables O b. all other answers are wrong O c. declare global variables O d. handle application events
-
Scuba divers carry ballast weights to have neutral buoyancy. At that condition, the buoyancy force on the diver exactly balances weight, and there is no tendency either to float toward the surface or...
-
Examine the transition between the laminar and turbulent flows of water by sketching the stream of water that exits from a faucet (without an aerator) or hose (without a nozzle). You can control the...
-
Water flows through a 5-cm-diameter pipe at the average velocity of 1.25 m/s. (a) In the dimensions of L/s, what is the volumetric flow rate? (b) If the diameter of the pipe is reduced by 20% at a...
-
A bank can either invest money for three months at 4.00% or for nine months at 4.50%. Ignoring actual/360 day count adjustments for the purpose of this question, the three against nine FRA quote the...
-
Shuggy Otis, an executive at Slapfish Corp. (SC) intends to retire in 11 years. SC just announced that it will start depositing $500.00 at the end of each quarter into each of its workers' retirement...
-
On January 1, the Hanover Beverage Company replaced the palletizing machine on one of its juice lines. The cost of the machine was $195,000. The machine's expected life is five years or 480,000...

Study smarter with the SolutionInn App


