Using activities, find the concentrations of the major species in 0.10 M NaClO 4 saturated with Mn(OH)
Question:
Using activities, find the concentrations of the major species in 0.10 M NaClO4 saturated with Mn(OH)2. Take the ionic strength to be 0.10 M and suppose that the ion size of MnOH+ is the same as Mn2+. Consider just the following chemistry:
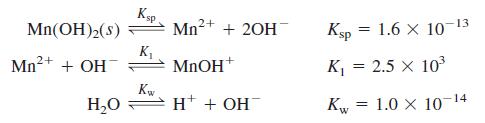
Transcribed Image Text:
Ksp Mn2+ 13 Mn(OH)2(s) + 20H Ksp = 1.6 X 10 Mn2+ + OH K, MNOH* K = 2.5 x 103 К H* + OH 1.0 x 10- Kw -14 H,0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
Or H Mn OHS Ksp Mn 2OH Or H Ksp 161013 Mn OHK Mn ...View the full answer

Answered By

Saurav Solanki
I love physics . After i did btech(grad) in electronics , started teaching physics as a home tutor , later in 2017 i satrted tutoring online. I have taught in Chegg , 24houranswers.com . Learning physics concept is easy when taught with practical examples . With my 3 yrs of experience , i want you to give me chance to teach you in a better way.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Use the systematic treatment of equilibrium to find the concentrations of the major species in a saturated aqueous solution of LiF. Consider these reactions: LiF(s) = Li* +F Ksp = [Li*]YLi+[F]YF Kjon...
-
Find the concentrations of Ag+(aq), NH3(aq), and [Ag(NH3)2]+(aq) at equilibrium when 0.10 mol Ag+(aq) and 0.10 mol NH3(aq) are made up to 1.00 L of solution. The dissociation constant, Kd, for the...
-
(a) Find the concentrations of species in saturated CaF 2 as a function of pH by using Reactions 12-32 through 12-36 and adding the following reaction: Do not include activity coefficients. Produce a...
-
At its headquarters in Ventura, California, Patagonia's office space feels more like a national park lodge than the main office of a $400 million retailer. It has a Douglas fir staircase and a...
-
A developer acquired a parcel of unimproved real property that she would like to develop. Although the land is currently zoned for commercial use, the developer would prefer not to begin development...
-
Oxygen boils at 183 C. The Fahrenheit equivalent of this temperature is a. 215 F b. 297 F c. 329 F d. 361 F
-
Explain the three types of visibility for package elements: public, private, and protected.
-
Shape It Manufacturing Company makes sheet metal products. For the past several years, the companys income has been declining. Its statements of cost of goods manufactured and income statements for...
-
1. Consider a situation where your company you are support technician at large corporation. An employee submits "ticket" reporting that they cannot access the internet. Upon further investigation you...
-
Using the same data set as in Exercise 3 and for house price growth, run several regression models with one, two, three, and four lags of price growth in the right-hand side of the model. Analyze the...
-
Write charge and mass balances for aqueous Ca 3 (PO 4 ) 2 if the species are Ca 2+ , CaOH + , CaPO - 4 , PO 3- 4 , HPO 2 4 - , H 2 PO - 4 , and H 3 PO 4 .
-
Explain why the solubility of an ionic compound increases as the ionic strength of the solution increases (at least up to ~ 0.5 M).
-
Identify key elements in the Universitys organisational field and explain the importance of these to the controversy.
-
Evaluate the following extension proposals; a. Bank of America into home safes b. Crest into a chain of dentist offices c. Caterpillar into automobiles d. Google into flight reservations
-
Given the regression estimate of the demand equation of where Y is income, what is the change in demand if price rises by $1, holding income constant? What is the percentage change in demand if price...
-
Now that you understand the difference between marginal costing and absorption costing, write a short evaluation of the two approaches.
-
Rich people consume more health care services than poor people. Explain two ways one might test this hypothesis.
-
Describe the size of the health economy when measured by the quantities of capital and labor used to produce health care. What important inputs to the production of health are not being counted among...
-
The Davis national drugstore chain prefers to operate one outlet in a town that has four major market segments. The number of potential customers in each segment along with the coordinates are as...
-
d. The characteristic equation of a control system is given by s+2s+8s+12s+20s+16+16=0. Determine the number of the roots of the equation which lie on the imaginary axis of s-plane
-
Calculate pCo2+ at each of the following points in the titration of 25.00 mL of 0.020 26 M Co2+ by 0.03855 M EDTA at pH 6.00: (a) 12.00 mL; (b) Ve; (c) 14.00 mL.
-
Consider the titration of 25.0 mL of 0.0200 M MnSO4 with 0.010 0 M EDTA in a solution buffered to pH 8.00. Calculate pMn2+ at the following volumes of added EDTA and sketch the titration curve: (a) 0...
-
For the same volumes used in Problem 11-8, calculate pCa2+ for the titration of 25.00 mL of 0.02000 M EDTA with 0.010 00 M CaSO4 at pH 10.00.
-
1) Explain how a Preschool program can help to bring a sense of belonging for the child? 2) It is important for a program to provide physical environments that are age-appropriate and that support...
-
Your company decided to organize a special dinner to appreciate all the employees and it has been proposed that the dinner to be held in the middle of December 2021. You have been appointed as the...
-
Select a two-dimensional image to analyze that is either a work of art (photograph, illustration, or painting) or an advertisement. What is the FTC analysis?Use the FTC palette to break down the...

Study smarter with the SolutionInn App


