The following reactions have the indicated equilibrium constants at a particular temperature: Determine the values of the
Fantastic news! We've Found the answer you've been seeking!
Question:
The following reactions have the indicated equilibrium constants at a particular temperature:
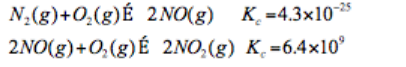
Determine the values of the equilibrium constants for the following equations at the same temperature:
a.) 4NO(g) N 2 (g)+2NO 2 (g)
b.) 4NO 2 (g) 2N 2 (g) + 4O 2 (g)
c.) 2NO(g)+2NO 2 (g) 3O2 2 (g)+2N 2 (g)
Related Book For 

Posted Date:




