Question: 06 Question (3 points) LAUNCH TUTORIAL LESSON e See page 660 COAST Tutorial Problem The rate of the reaction NO2(g) + CO(g) NO(g) + CO2(8)
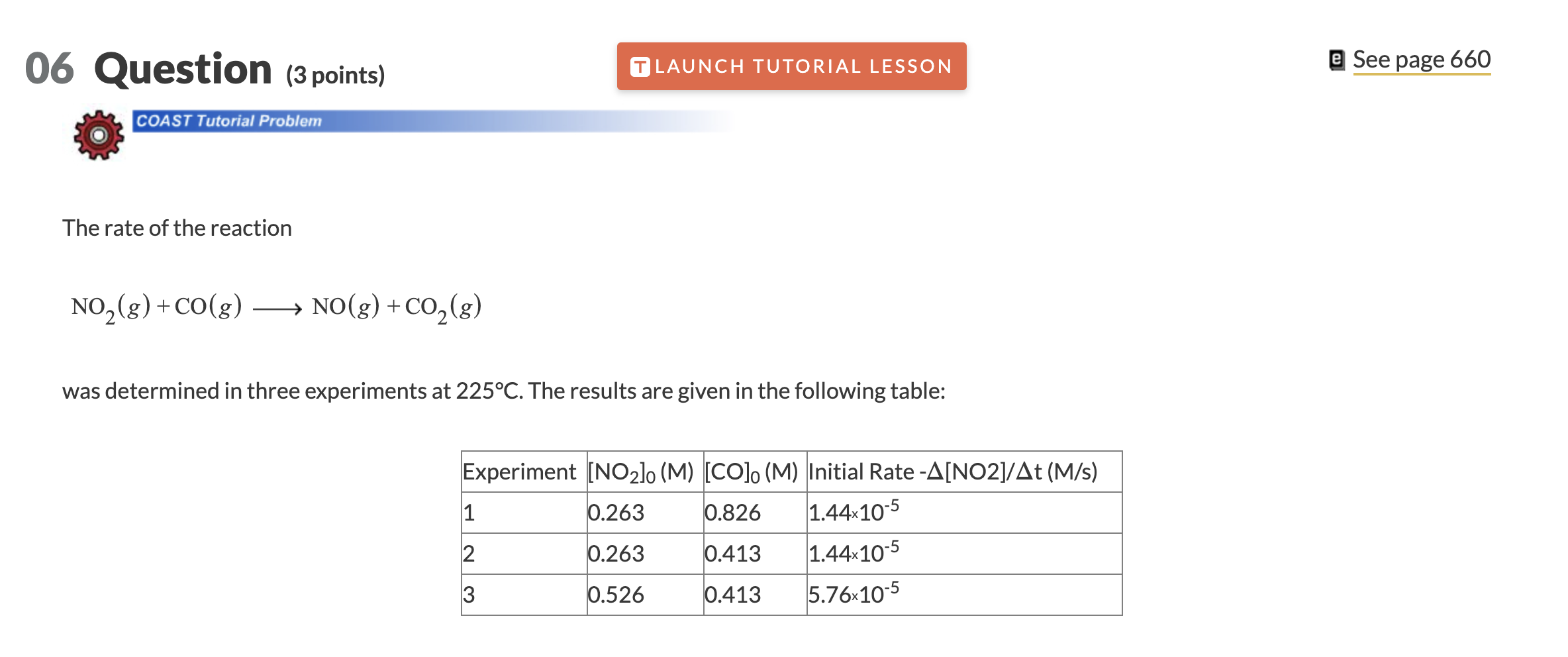
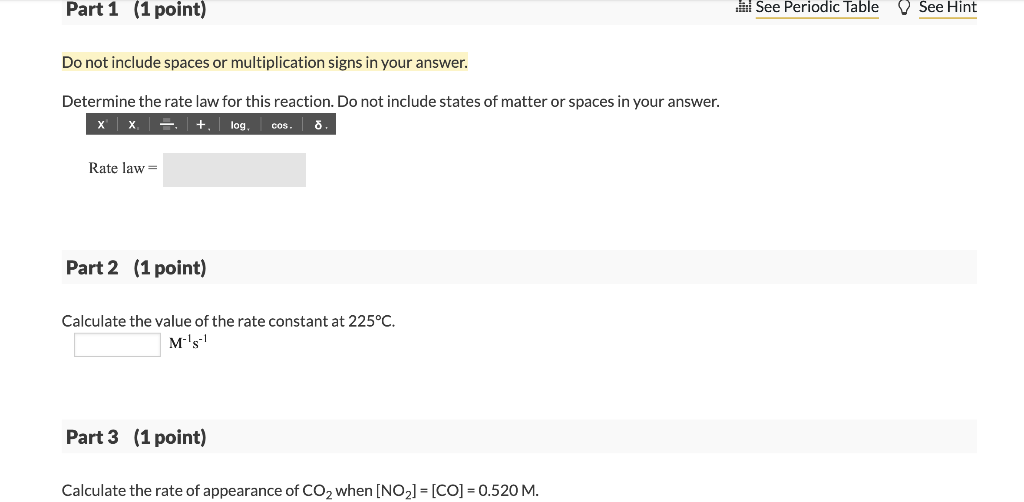
06 Question (3 points) LAUNCH TUTORIAL LESSON e See page 660 COAST Tutorial Problem The rate of the reaction NO2(g) + CO(g) NO(g) + CO2(8) ) g was determined in three experiments at 225C. The results are given in the following table: Experiment [NO2lo (M) [CO]. (M) Initial Rate -A[NO2]/At (M/s) 1 0.263 0.826 1.44x10-5 2 0.263 0.413 1.44x10-5 3 0.526 0.413 5.76x10-5 I Part 1 (1 point) il See Periodic Table See Hint Do not include spaces or multiplication signs in your answer. Determine the rate law for this reaction. Do not include states of matter or spaces in your answer. x x + log, o cos. Rate law= Part 2 (1 point) Calculate the value of the rate constant at 225C. M's-1 Part 3 (1 point) Calculate the rate of appearance of CO2 when [NO2] = [CO] = 0.520 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


