Question: 09 Question (2 points) T LAUNCH TUTORIAL LESSON @ See page 768 COAST Tutorial Problem Calculate the pH at 25C of 240.0 mL of a
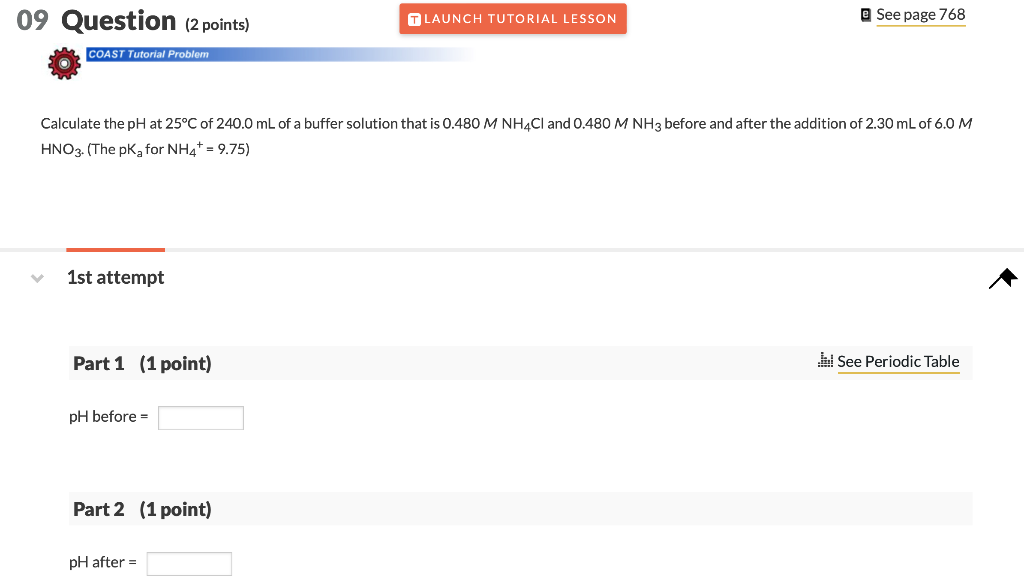
09 Question (2 points) T LAUNCH TUTORIAL LESSON @ See page 768 COAST Tutorial Problem Calculate the pH at 25C of 240.0 mL of a buffer solution that is 0.480 M NH4Cl and 0.480 M NH3 before and after the addition of 2.30 mL of 6.0 M HNO3. (The pka for NH4+ = 9.75) 1st attempt Part 1 (1 point) Il See Periodic Table pH before = Part 2 (1 point) pH after =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


