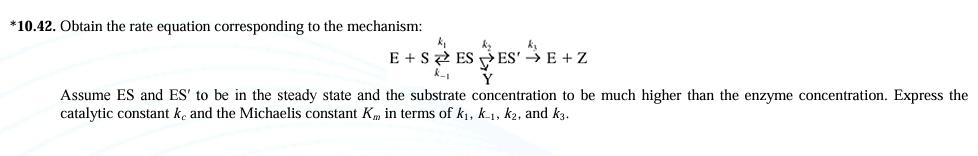
Question: * 1 0 . 4 2 . Obtain the rate equation corresponding to the mechanism: E + S k - 1 k 1 E S
Obtain the rate equation corresponding to the mechanism:
Assume ES and to be in the steady state and the substrate concentration to be much higher than the enzyme concentration. Express the catalytic constant and the Michaelis constant in terms of and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


