Question: 1 1 . 8 Nitrogen dioxide, N O 2 , produced by a thermal process for fixation of nitrogen is to be removed from a
Nitrogen dioxide, produced by a thermal process for fixation of nitrogen is to be removed
from a dilute mixture with air by adsorption on silica gel in a continuous countercurrent adsorber.
The gas entering the adsorber at the rate of contains by volume, and of
the is to be removed. Operation is to be isothermal at std atm. The entering gel will be
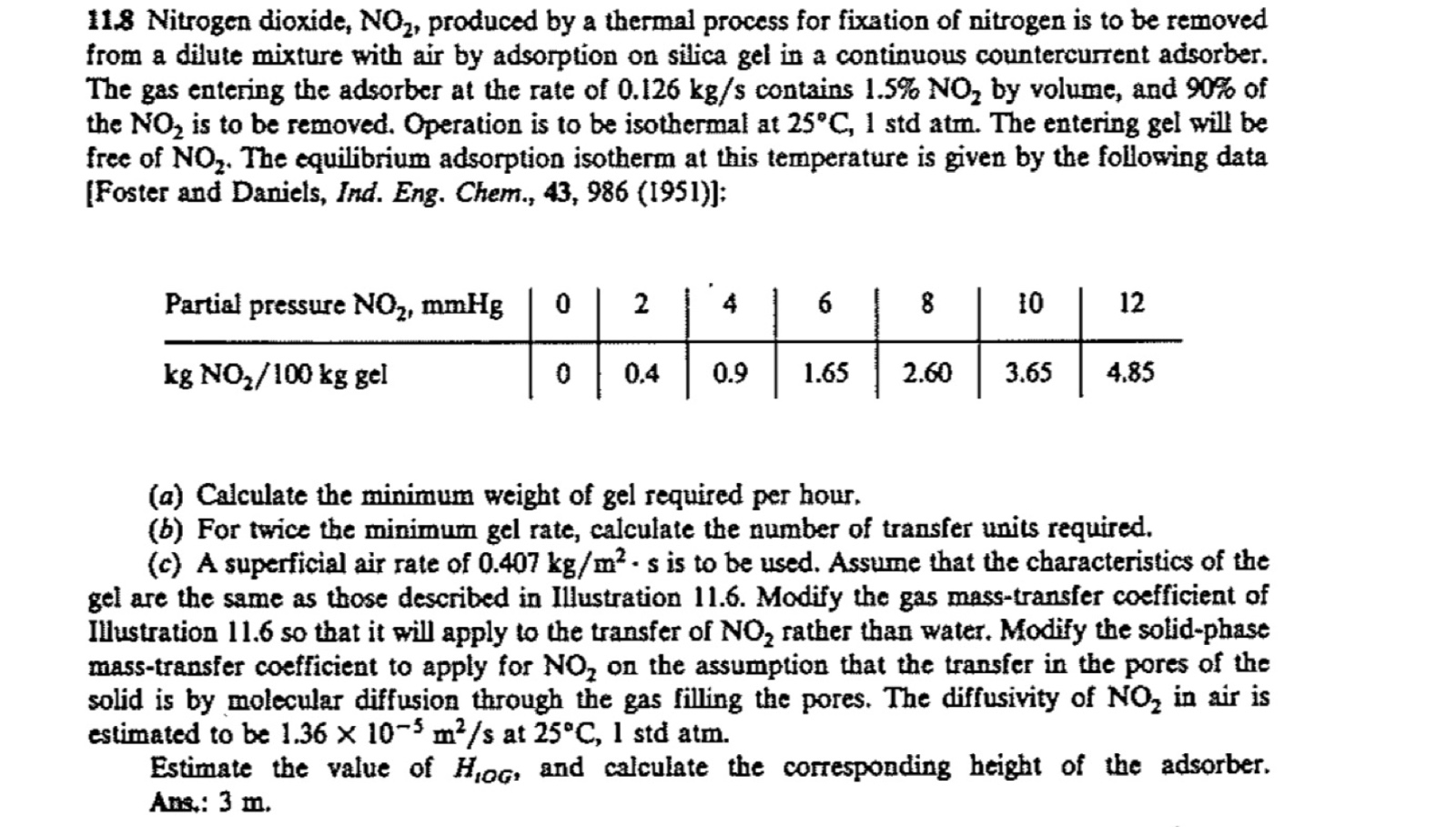
free of The equilibrium adsorption isotherm at this temperature is given by the following data
Foster and Daniels, Ind. Eng. Chem., ;
a Calculate the minimum weight of gel required per hour.
b For twice the minimum gel rate, calculate the number of transfer units required.
c A superficial air rate of is to be used. Assume that the characteristics of the
gel are the same as those described in Illustration Modify the gas masstransfer coefficient of
Illustration so that it will apply to the transfer of rather than water. Modify the solidphase
masstransfer coefficient to apply for on the assumption that the transfer in the pores of the
solid is by molecular diffusion through the gas filling the pores. The diffusivity of in air is
estimated to be at std atm.
Estimate the value of and calculate the corresponding height of the adsorber.
Ams:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


