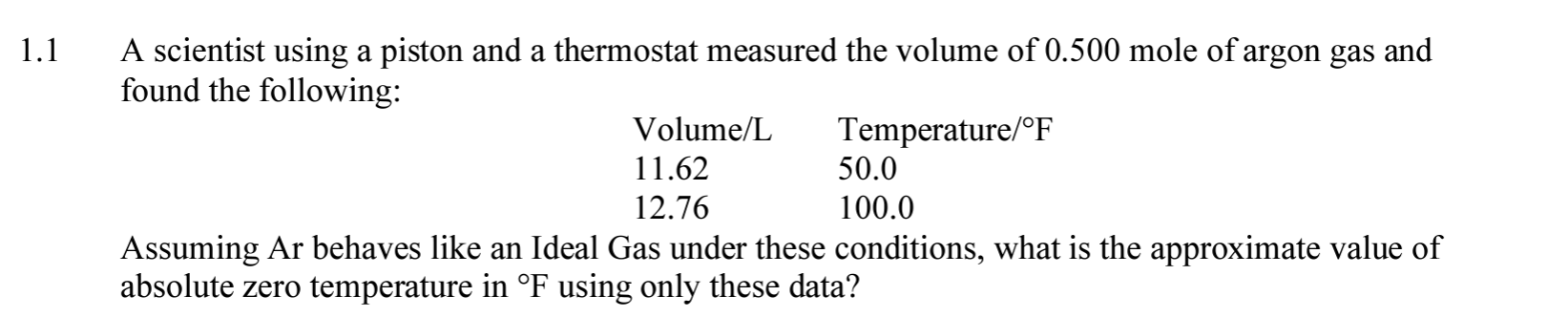
Question: 1 . 1 A scientist using a piston and a thermostat measured the volume of 0 . 5 0 0 mole of argon gas and
A scientist using a piston and a thermostat measured the volume of mole of argon gas and
found the following:
Assuming Ar behaves like an Ideal Gas under these conditions, what is the approximate value of
absolute zero temperature in using only these data?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


