Question: PLEASE SOLVE COMPLETELY WITHIN 4 HOURS! Please do not copy and paste, I will upvote! Thank you (: Ai Problem 3. The performance of an
PLEASE SOLVE COMPLETELY WITHIN 4 HOURS! Please do not copy and paste, I will upvote! Thank you (:
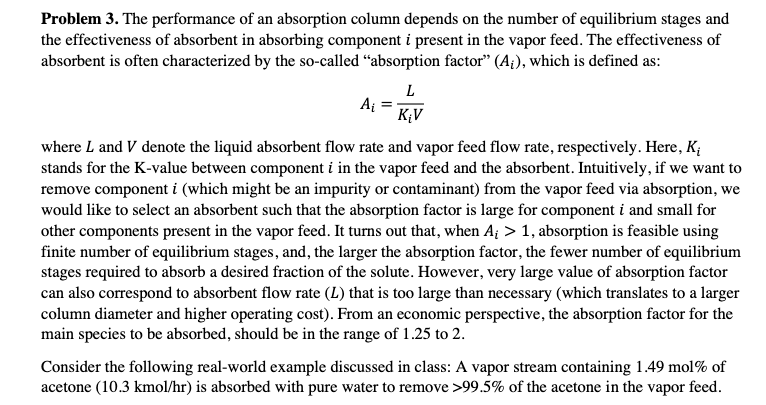
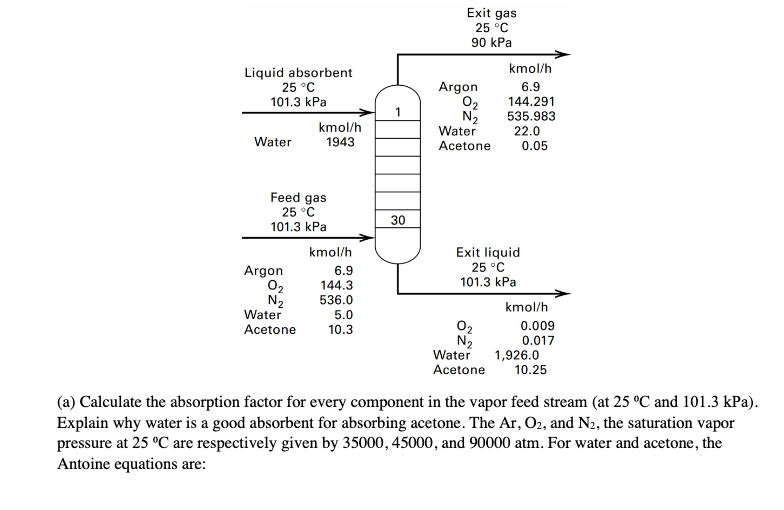

Ai Problem 3. The performance of an absorption column depends on the number of equilibrium stages and the effectiveness of absorbent in absorbing component i present in the vapor feed. The effectiveness of absorbent is often characterized by the so-called "absorption factor (A;), which is defined as: L KV where L and V denote the liquid absorbent flow rate and vapor feed flow rate, respectively. Here, K; stands for the K-value between component i in the vapor feed and the absorbent. Intuitively, if we want to remove component i (which might be an impurity or contaminant) from the vapor feed via absorption, we would like to select an absorbent such that the absorption factor is large for component i and small for other components present in the vapor feed. It turns out that, when Ai > 1, absorption is feasible using finite number of equilibrium stages, and, the larger the absorption factor, the fewer number of equilibrium stages required to absorb a desired fraction of the solute. However, very large value of absorption factor can also correspond to absorbent flow rate (L) that is too large than necessary (which translates to a larger column diameter and higher operating cost). From an economic perspective, the absorption factor for the main species to be absorbed, should be in the range of 1.25 to 2. Consider the following real-world example discussed in class: A vapor stream containing 1.49 mol% of acetone (10.3 kmol/hr) is absorbed with pure water to remove >99.5% of the acetone in the vapor feed. Liquid absorbent 25 C 101.3 kPa kmol/h Water 1943 Exit gas 25 C 90 kPa kmol/h Argon 6.9 02 144.291 N2 535.983 Water 22.0 Acetone 0.05 30 Argon Feed gas 25C 101.3 kPa kmol/h 6.9 02 144.3 N2 536.0 Water 5.0 Acetone 10.3 Exit liquid 25 C 101.3 kPa kmol/h 0.009 N2 0.017 Water 1,926.0 Acetone 10.25 02 (a) Calculate the absorption factor for every component in the vapor feed stream (at 25 C and 101.3 kPa). Explain why water is a good absorbent for absorbing acetone. The Ar, O2, and N2, the saturation vapor pressure at 25 C are respectively given by 35000, 45000, and 90000 atm. For water and acetone, the Antoine equations are: 1838.675 T-31.737 1312.253 T-32.445 For water: log Pesat = 5.40221 For acetone: log Paart acetone = 4.42448 where psat is in bar and T is in K. Since water is also used as the absorbent, Yh20 = 1. The activity coefficient for acetone is determined to be 6.7 at 25 C and 101.3 kPa. (b) Use Aspen HYSYS to simulate this absorption process (input information about the vapor feed and liquid absorbent feed, and let Aspen solve for both liquid and gas products). Use UNIQUAC thermodynamic package in your simulation. Include a screenshot showing the converged flowsheet, as well as another screenshot showing component molar flow rates of both exiting streams, which can be found under the Performance tab by double clicking the absorber in the simulation environment. (c) If we fix the vapor feed stream composition and flow rate, but increase the absorbent feed flow rate by 50%, or 1.5 x 1943 = 2914.5 kmol/hr, determine the acetone and N2 mole fractions in exiting vapor and liquid streams, assuming the column has 30 theoretical stages (i.e., stage efficiency = 100%). State any assumptions used when solving this problem. Hint: Use the Kremser equation. (d) If the compositions of the vapor feed stream and absorbent feed stream are unchanged, but we simultaneously double the flow rate in both vapor feed and absorbent feed streams, how will the acetone composition change in exiting vapor and liquid streams? Why? Hint: Detailed calculations are not needed for answering this
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


