Question: 1) 2.) Draw a Lewis structure for the CH4O molecule, using the connectivity shown in the spacefilling model in the window. (Gray = C; white
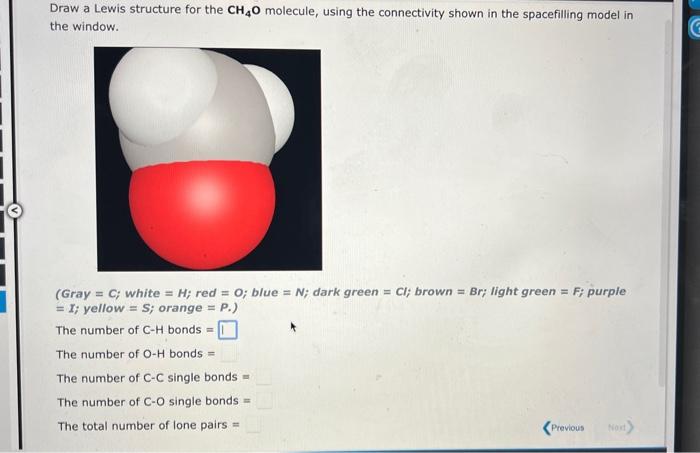
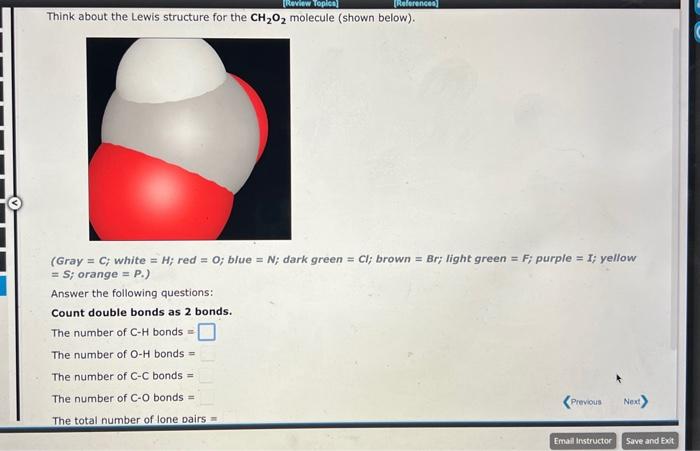
Draw a Lewis structure for the CH4O molecule, using the connectivity shown in the spacefilling model in the window. (Gray = C; white = H; red =0; blue =N; dark green = Cl; brown = Br; light green = F; purple = I; yellow = S; orange = P.) The number of CH bonds = The number of OH bonds = The number of CC single bonds = The number of CO single bonds = The total number of lone pairs = Think about the Lewis structure for the CH2O2 molecule (shown below). (Gray =C;white =H; red =0; blue =N; dark green =C; brown = Br; light green = F; purple =I; yellow = S; orange = P.) Answer the following questions: Count double bonds as 2 bonds. The number of CH bonds = The number of OH bonds = The number of CC bonds = The number of CO bonds = The total number of lone Dairs =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


