Question: 1 3 . 6 . In Example 1 3 . 3 we assumed that all the HC was combusted, and thus all the oxygen deficit
In Example we assumed that all the HC was combusted, and thus all the oxygen deficit
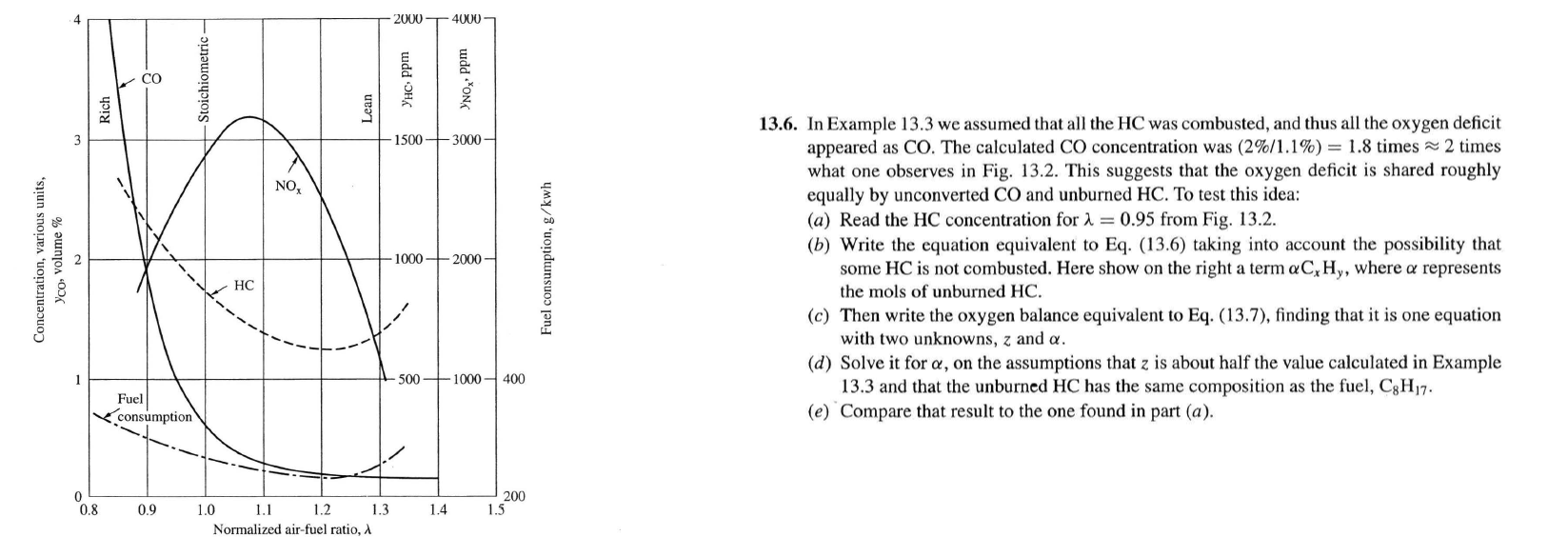
appeared as CO The calculated CO concentration was times ~~ times
what one observes in Fig. This suggests that the oxygen deficit is shared roughly
equally by unconverted CO and unburned HC To test this idea:
a Read the HC concentration for lambda from Fig.
b Write the equation equivalent to Eq taking into account the possibility that
some HC is not combusted. Here show on the right a term alpha CxHy where alpha represents
the mols of unburned HC
c Then write the oxygen balance equivalent to Eq finding that it is one equation
with two unknowns, z and alpha
d Solve it for alpha on the assumptions that z is about half the value calculated in Example
and that the unburned HC has the same composition as the fuel, CH
e Compare that result to the one found in part a
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


