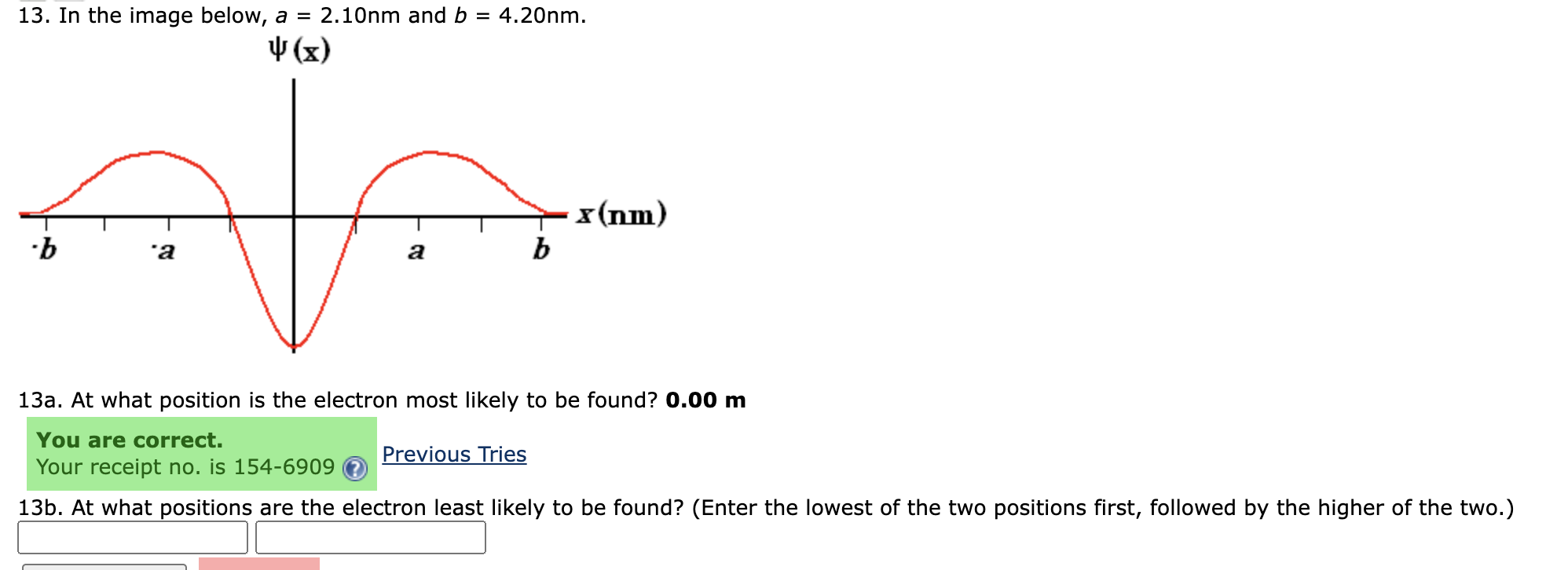
Question: 1 3 . In the image below, ( a = 2 . 1 0 mathrm { ~nm } ) and (
In the image below, amathrm~nm and bmathrm~nm
Ill
a At what position is the electron most likely to be found? mathbfmathbf~ m
You are correct.
Your receipt no is Previous Tries
b At what positions are the electron least likely to be found? Enter the lowest of the two positions first, followed by the higher of the two.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


