Question: 1. (4 pts) You will need the following data table for this lab. This was made in Excel, so if you want Excel to do
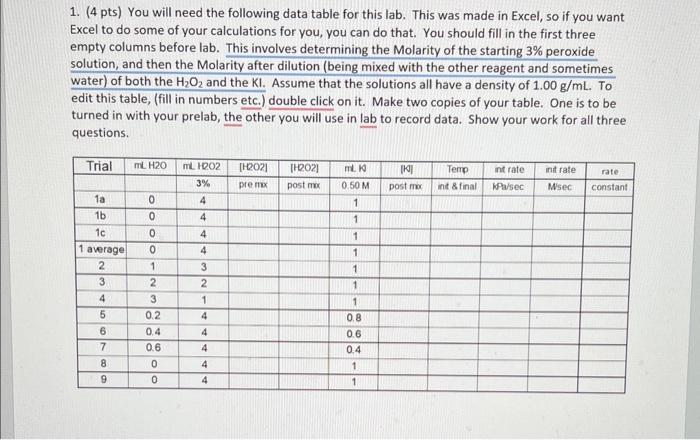
1. (4 pts) You will need the following data table for this lab. This was made in Excel, so if you want Excel to do some of your calculations for you, you can do that. You should fill in the first three empty columns before lab. This involves determining the Molarity of the starting 3% peroxide solution, and then the Molarity after dilution (being mixed with the other reagent and sometimes water) of both the H2O2 and the KI. Assume that the solutions all have a density of 1.00g/mL. To edit this table, (fill in numbers etc.) double click on it. Make two copies of your table. One is to be turned in with your prelab, the other you will use in lab to record data. Show your work for all three questions. 1. (4 pts) You will need the following data table for this lab. This was made in Excel, so if you want Excel to do some of your calculations for you, you can do that. You should fill in the first three empty columns before lab. This involves determining the Molarity of the starting 3% peroxide solution, and then the Molarity after dilution (being mixed with the other reagent and sometimes water) of both the H2O2 and the KI. Assume that the solutions all have a density of 1.00g/mL. To edit this table, (fill in numbers etc.) double click on it. Make two copies of your table. One is to be turned in with your prelab, the other you will use in lab to record data. Show your work for all three questions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


