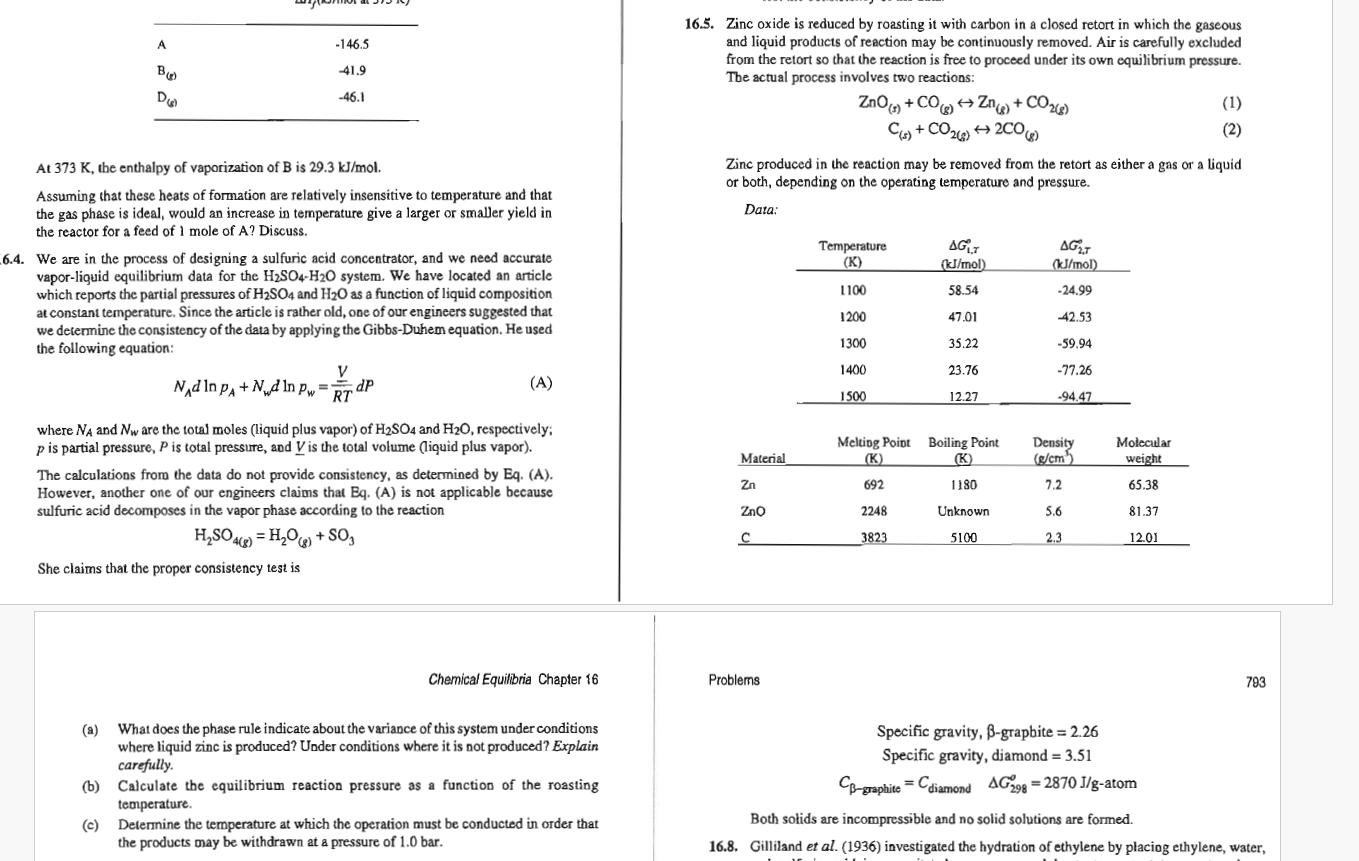
Question: 1 6 . 5 ONLy 1 6 . 5 . Zinc oxide is reduced by roasting it with carbon in a closed retort in which
ONLy
Zinc oxide is reduced by roasting it with carbon in a closed retort in which the gaseous
and liquid products of reaction may be continuously removed. Air is carefully excluded
from the retort so that the reaction is free to proceed under its own equilibrium pressure.
The actual process involves two reactions:
harr
Zinc produced in the reaction may be removed from the retort as either a gas or a liquid
or both, depending on the operating temperature and pressure.
Data:
She claims that the proper consistency test is
a What does the phase rule indicate about the variance of this system under conditions
where liquid zinc is produced? Under conditions where it is not produced? Explain
carefully.
b Calculate the equilibrium reaction pressure as a function of the roasting
temperature.
c Determine the temperature at which the operation must be conducted in order that
the products may be withdrawn at a pressure of bar.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


