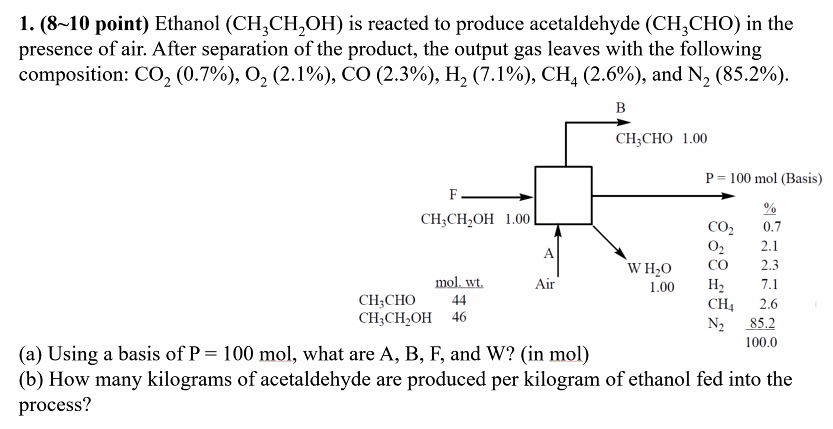
Question: 1 . ( 8 1 0 point ) Ethanol ( CH _ 3 CH _ 2 OH ) is reacted to produce acetaldehyde ( CH
point Ethanol CHCHOH is reacted to produce acetaldehyde CHCHO
point Ethanol is reacted to produce acetaldehyde CHO in the presence of air. After separation of the product, the output gas leaves with the following composition: and
:
a Using a basis of mol, what are and in mol
b How many kilograms of acetaldehyde are produced per kilogram of ethanol fed into the process?in the presence of air. After separation of the product, the output gas leaves with the following composition: CO
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


