Question: 1. A 519-g empty iron kettle is put on a stove. How much heat, in joules, must it absorb to raise its temperature from 13.0C
1.
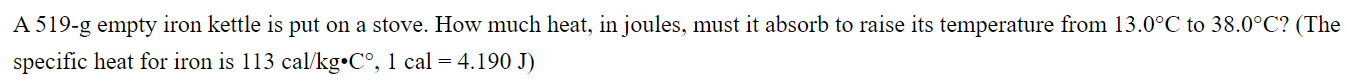



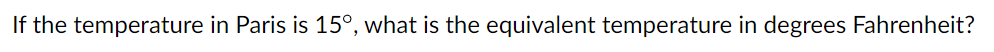
A 519-g empty iron kettle is put on a stove. How much heat, in joules, must it absorb to raise its temperature from 13.0C to 38.0C? (The specific heat for iron is 113 cal/kg C, 1 cal = 4.190 J)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


