Question: 1. A) Determine if the molecules below are chiral or achiral. CH3 OH & ha ly NH2 B) Draw the enantiomers of the chiral molecules
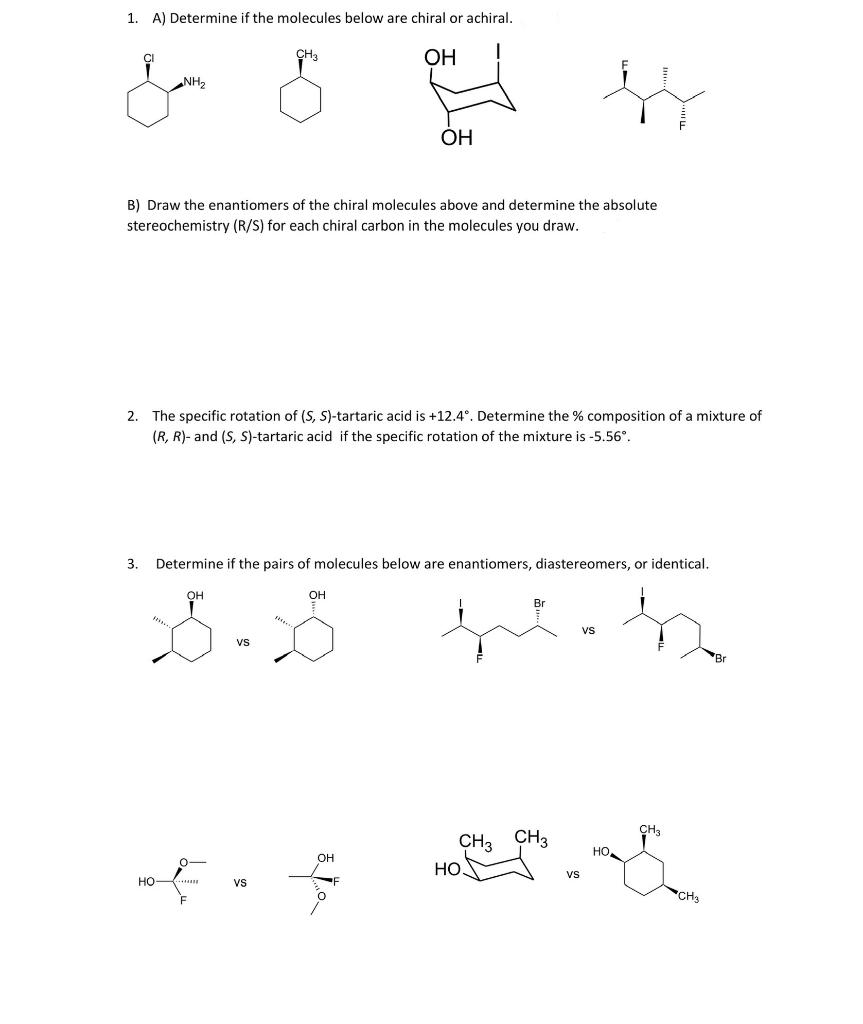
1. A) Determine if the molecules below are chiral or achiral. CH3 OH & ha ly NH2 B) Draw the enantiomers of the chiral molecules above and determine the absolute stereochemistry (R/S) for each chiral carbon in the molecules you draw. 2. The specific rotation of (S, S)-tartaric acid is +12.4. Determine the % composition of a mixture of (R, R)- and (S, S)-tartaric acid if the specific rotation of the mixture is -5.56. 3 Determine if the pairs of molecules below are enantiomers, diastereomers, or identical. OH OH Br VS VS CH3 CH3 CH3 , OH HO VS HO VS ote CH3
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


