Question: visualizing molecular chirality using cholesteric liquid crystals: write a mechanism and balanced equations and theoretical yield for all reactions Visualizing molecular chirality in the organic

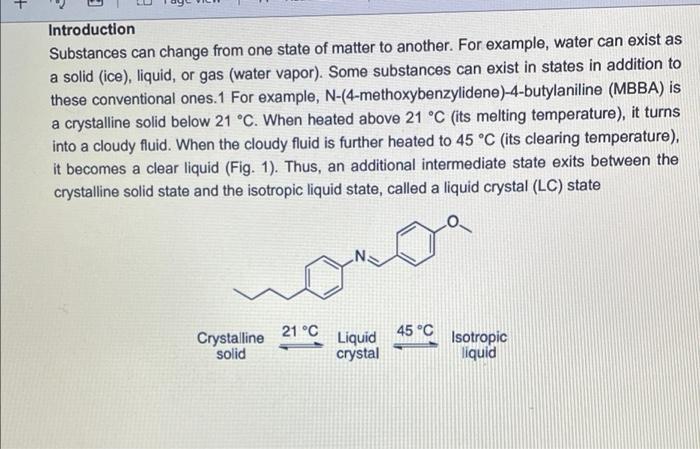
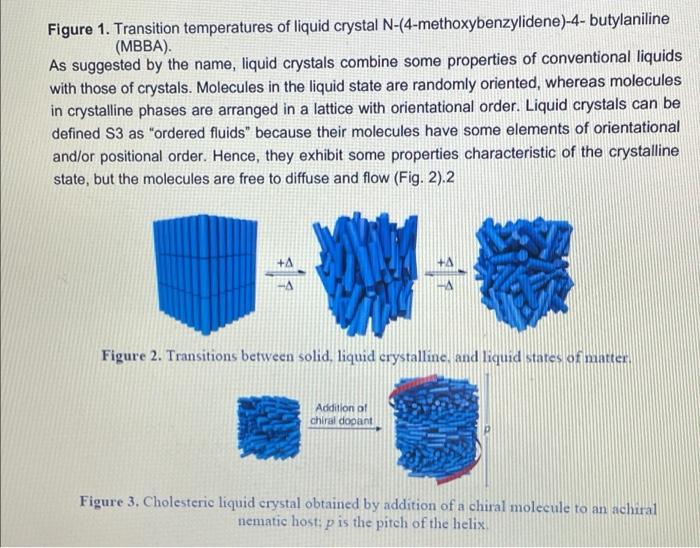
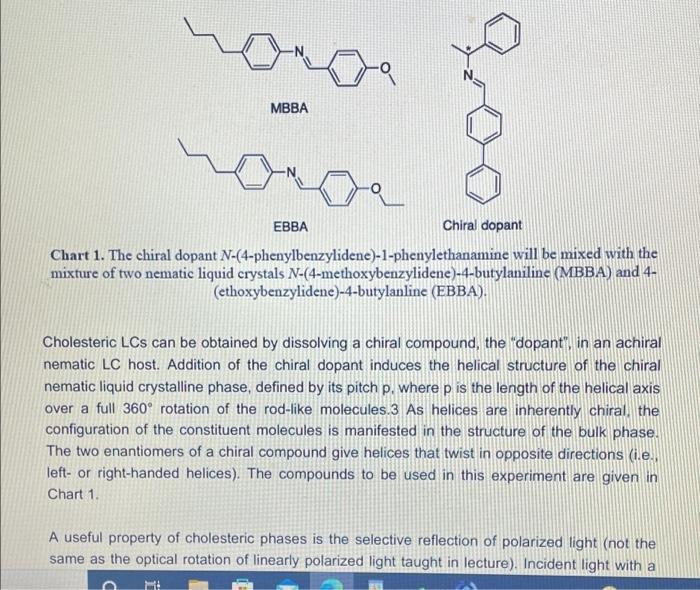

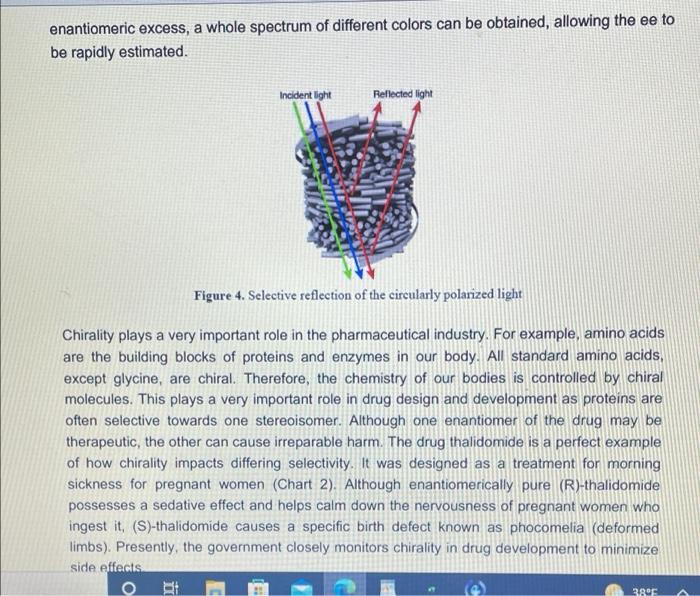
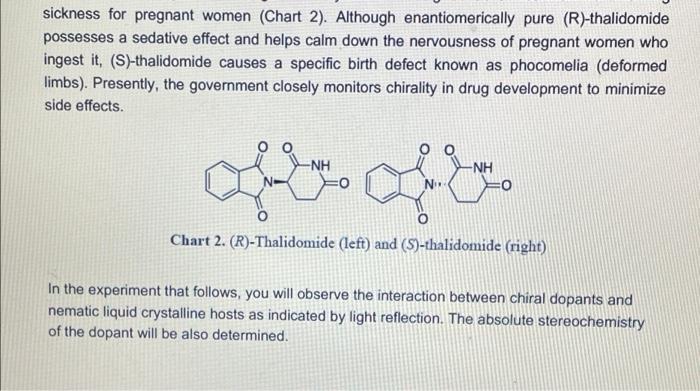
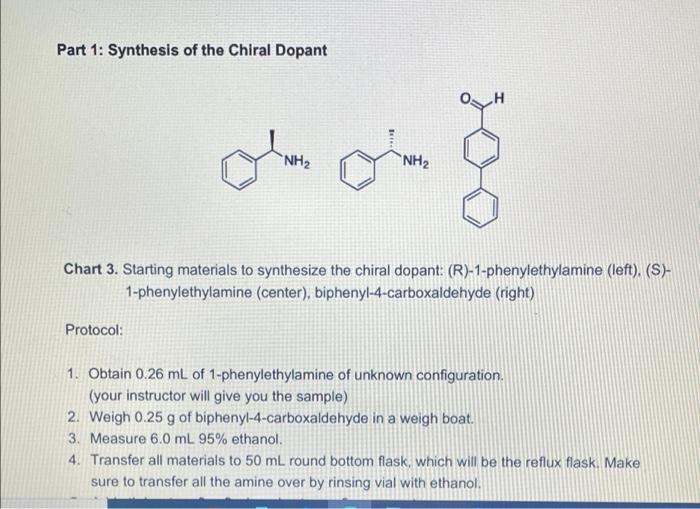

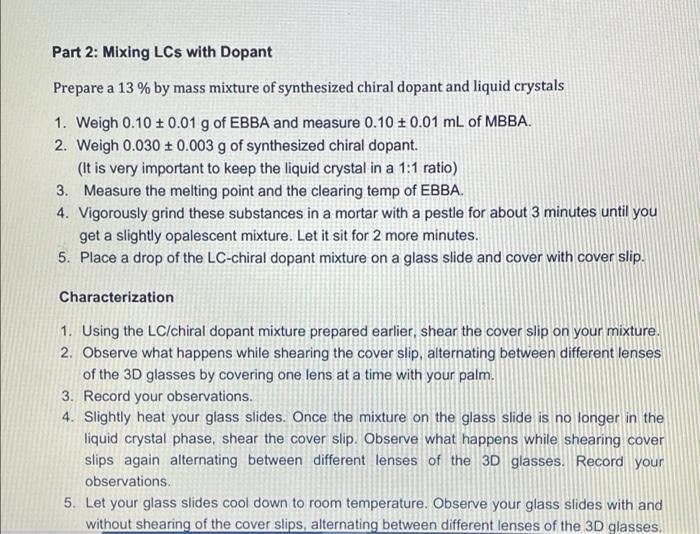

Visualizing molecular chirality in the organic chemistry laboratory using cholesteric liquid crystals Objectives To investigate chirality by observing its macroscopic manifestations To synthesize an imine To determine the absolute stereochemistry of a product mixture To examine liquid crystals and their phase behavior To identify a structure-property relationship between chiral dopants and liquid crystals . . . . Safety 1-phenylethylamine is corrosive. The toxicity of the synthesized chiral imine is unknown. Many imines are carcinogenic. Biphenyl-4-carboxaldehyde, ethanol, MBBA, and EBBA are irritants to the skin, eyes, and respiratory system. Wear gloves during all operations involving the chiral dopant. The synthesis should be conducted in a fume hood. . Introduction Substances can change from one state of matter to another. For example, water can exist as a solid (ice), liquid, or gas (water vapor). Some substances can exist in states in addition to these conventional ones. 1 For example, N-(4-methoxybenzylidene)-4-butylaniline (MBBA) is a crystalline solid below 21 C. When heated above 21 C (its melting temperature), it turns BI 38er + Introduction Substances can change from one state of matter to another. For example, water can exist as a solid (ice), liquid, or gas (water vapor). Some substances can exist in states in addition to these conventional ones. For example, N-(4-methoxybenzylidene)-4-butylaniline (MBBA) is a crystalline solid below 21 C. When heated above 21 C (its melting temperature), it turns into a cloudy fluid. When the cloudy fluid is further heated to 45 C (its clearing temperature), it becomes a clear liquid (Fig. 1). Thus, an additional intermediate state exits between the crystalline solid state and the isotropic liquid state, called a liquid crystal (LC) state 21 C 45C Crystalline solid Liquid crystal Isotropic liquid Figure 1. Transition temperatures of liquid crystal N-(4-methoxybenzylidene)-4-butylaniline (MBBA). As suggested by the name, liquid crystals combine some properties of conventional liquids with those of crystals. Molecules in the liquid state are randomly oriented, whereas molecules in crystalline phases are arranged in a lattice with orientational order. Liquid crystals can be defined S3 as "ordered fluids" because their molecules have some elements of orientational and/or positional order. Hence, they exhibit some properties characteristic of the crystalline state, but the molecules are free to diffuse and flow (Fig. 2).2 Figure 2. Transitions between solid, liquid crystalline and liquid states of marter Additional chiral dopant Figure 3. Cholesteric liquid crystal obtained by addition of a chiral molecule to an achiral nematic host: p is the pitch of the helix MBBA EBBA Chiral dopant Chart 1. The chiral dopant N-(4-phenylbenzylidene)-1-phenylethanamine will be mixed with the mixture of two nematic liquid crystals N-(4-methoxybenzylidene)-4-butylaniline (MBBA) and 4- (ethoxybenzylidene)-4-butylanline (EBBA). Cholesteric LCs can be obtained by dissolving a chiral compound, the "dopant", in an achiral nematic LC host. Addition of the chiral dopant induces the helical structure of the chiral nematic liquid crystalline phase, defined by its pitch p, where p is the length of the helical axis over a full 360 rotation of the rod-like molecules.3 As helices are inherently chiral, the configuration of the constituent molecules is manifested in the structure of the bulk phase. The two enantiomers of a chiral compound give helices that twist in opposite directions (ie. left- or right-handed helices). The compounds to be used in this experiment are given in Chart 1 A useful property of cholesteric phases is the selective reflection of polarized light (not the same as the optical rotation of linearly polarized light taught in lecture). Incident light with a A useful property of cholesteric phases is the selective reflection of polarized light (not the same as the optical rotation of linearly polarized light taught in lecture). Incident light with a S4 wavelength similar to the pitch (p) of the helix is reflected when it strikes a film of a cholesteric liquid crystal. The observed color therefore depends on the pitch of the helix. The reflected light has another important property: it is circularly polarized, with the polarization dependent on the twist sense of the cholesteric helix. The material reflects only circular polarized light with the same handedness as the handedness of the helix. The opposite handedness is transmitted through the material. In other words, a left-handed helix will selectively reflect left-handed circularly polarized light. This combination of properties has found broad application in the production of liquid crystal thermometers. This method can also be used for enantiomeric excess (ee) determination by visual color inspection. The method makes use of the sensitivity of the pitch towards the strength of the chiral perturbation. A chiral analyte of interest is doped into the liquid crystal, and, based on its enantiomeric excess, a whole spectrum of different colors can be obtained, allowing the ee to be rapidly estimated Incident light Reflected light Figure 4. Selective reflection of the circularly polarized light Chirality plays a very important role in the pharmaceutical industry. For example, amino acids are the building blocks of proteins and enzymes in our body. All standard amino acids, except glycine, are chiral. Therefore, the chemistry of our bodies is controlled by chiral molecules. This plays a very important role in drug design and development as proteins are often selective towards one stereoisomer. Although one enantiomer of the drug may be therapeutic, the other can cause irreparable harm. The drug thalidomide is a perfect example of how chirality impacts differing selectivity. It was designed as a treatment for morning sickness for pregnant women (Chart 2). Although enantiomerically pure (R)-thalidomide possesses a sedative effect and helps calm down the nervousness of pregnant women who ingest it. (S)-thalidomide causes a specific birth defect known as phocomelia (deformed limbs). Presently, the government closely monitors chirality in drug development to minimize side effects . 3.8C sickness for pregnant women (Chart 2). Although enantiomerically pure (R)-thalidomide possesses a sedative effect and helps calm down the nervousness of pregnant women who ingest it, (S)-thalidomide causes a specific birth defect known as phocomelia (deformed limbs). Presently, the government closely monitors chirality in drug development to minimize side effects. o ofte gore -NH 0 Chart 2. (R)-Thalidomide (left) and (S)-thalidomide (right) In the experiment that follows, you will observe the interaction between chiral dopants and nematic liquid crystalline hosts as indicated by light reflection. The absolute stereochemistry of the dopant will be also determined. Part 1: Synthesis of the Chiral Dopant NH2 NH2 Chart 3. Starting materials to synthesize the chiral dopant: (R)-1-phenylethylamine (left). (S)- 1-phenylethylamine (center), biphenyl-4-carboxaldehyde (right) Protocol: 1. Obtain 0.26 mL of 1-phenylethylamine of unknown configuration (your instructor will give you the sample) 2. Weigh 0.25 g of biphenyl-4-carboxaldehyde in a weigh boat. 3. Measure 6.0 mL 95% ethanol. 4. Transfer all materials to 50 mL round bottom flask, which will be the reflux flask. Make sure to transfer all the amine over by rinsing vial with ethanol. Protocol: 1. Obtain 0.26 mL of 1-phenylethylamine of unknown configuration. (your instructor will give you the sample) 2. Weigh 0.25 g of biphenyl-4-carboxaldehyde in a weigh boat. 3. Measure 6.0 mL 95% ethanol. 4. Transfer all materials to 50 mL round bottom flask, which will be the reflux flask. Make sure to transfer all the amine over by rinsing vial with ethanol. 5. Add stir bar to reflux flask and set up reflux apparatus. 6. Heat at reflux for 15 minutes once the mixture starts boiling. 7. Measure about 7 mL of 95% ethanol into a small graduated cylinder and place graduated cylinder in beaker filled with ice. 8. After 15 minutes of boiling, take heat off and allow the solution to cool to room temperature. 9. Place the cooled flask into beaker filled with ice. 10. Collect crystals by vacuum filtration through a Buchner funnel. 11. Wash crystals with no more than 7 mL total of ice cold ethanol. 12. Leave on Buchner funnel, when crystals are completely dried, collect them into a previously tared weigh boat and determine mass of sample. 13. Obtain melting point and IR spectrum. Part 2: Mixing LCs with Dopant Prepare a 13 % by mass mixture of synthesized chiral dopant and liquid crystals 1. Weigh 0.10 0.01 g of EBBA and measure 0.10 + 0.01 mL of MBBA. 2. Weigh 0.030 + 0.003 g of synthesized chiral dopant. (It is very important to keep the liquid crystal in a 1:1 ratio) 3. Measure the melting point and the clearing temp of EBBA. 4. Vigorously grind these substances in a mortar with a pestle for about 3 minutes until you get a slightly opalescent mixture. Let it sit for 2 more minutes. 5. Place a drop of the LC-chiral dopant mixture on a glass slide and cover with cover slip. Characterization 1. Using the LC/chiral dopant mixture prepared earlier, shear the cover slip on your mixture. 2. Observe what happens while shearing the cover slip, alternating between different lenses of the 3D glasses by covering one lens at a time with your palm. 3. Record your observations. 4. Slightly heat your glass slides. Once the mixture on the glass slide is no longer in the liquid crystal phase, shear the cover slip. Observe what happens while shearing cover slips again alternating between different lenses of the 3D glasses. Record your observations 5. Let your glass slides cool down to room temperature. Observe your glass slides with and without shearing of the cover slips, alternating between different lenses of the 3D glasses, Characterization 1. Using the LC/chiral dopant mixture prepared earlier, shear the cover slip on your mixture. 2. Observe what happens while shearing the cover slip, alternating between different lenses of the 3D glasses by covering one lens at a time with your palm. 3. Record your observations. 4. Slightly heat your glass slides. Once the mixture on the glass slide is no longer in the liquid crystal phase, shear the cover slip. Observe what happens while shearing cover slips again alternating between different lenses of the 3D glasses. Record your observations. 5. Let your glass slides cool down to room temperature. Observe your glass slides with and without shearing of the cover slips, alternating between different lenses of the 3D glasses. Record your observations. 6. Obtain two glass slides from your instructor, one with known (R)-dopant in the mixture and one with the (S)-dopant. Shear the glass slides in the same way as you did for the glass slides with your LC/dopant mixtures and with the racemic mixture. Record your observations for each lens of the 3D glasses, 7. Now with each lens calibrated, determine which enantiomer you were assigned 8. Find a classmate who has the enantiomer of your compound and make a 13% racemic dopant-liquid crystals mixture using both of your samples. Place a drop of this mixture on a glass slide. Cover with a cover slip. Shear, heat, and cool down the glass slide in the same way as you did for the glass slides with your LC/chiral dopant mixtures, Visualizing molecular chirality in the organic chemistry laboratory using cholesteric liquid crystals Objectives To investigate chirality by observing its macroscopic manifestations To synthesize an imine To determine the absolute stereochemistry of a product mixture To examine liquid crystals and their phase behavior To identify a structure-property relationship between chiral dopants and liquid crystals . . . . Safety 1-phenylethylamine is corrosive. The toxicity of the synthesized chiral imine is unknown. Many imines are carcinogenic. Biphenyl-4-carboxaldehyde, ethanol, MBBA, and EBBA are irritants to the skin, eyes, and respiratory system. Wear gloves during all operations involving the chiral dopant. The synthesis should be conducted in a fume hood. . Introduction Substances can change from one state of matter to another. For example, water can exist as a solid (ice), liquid, or gas (water vapor). Some substances can exist in states in addition to these conventional ones. 1 For example, N-(4-methoxybenzylidene)-4-butylaniline (MBBA) is a crystalline solid below 21 C. When heated above 21 C (its melting temperature), it turns BI 38er + Introduction Substances can change from one state of matter to another. For example, water can exist as a solid (ice), liquid, or gas (water vapor). Some substances can exist in states in addition to these conventional ones. For example, N-(4-methoxybenzylidene)-4-butylaniline (MBBA) is a crystalline solid below 21 C. When heated above 21 C (its melting temperature), it turns into a cloudy fluid. When the cloudy fluid is further heated to 45 C (its clearing temperature), it becomes a clear liquid (Fig. 1). Thus, an additional intermediate state exits between the crystalline solid state and the isotropic liquid state, called a liquid crystal (LC) state 21 C 45C Crystalline solid Liquid crystal Isotropic liquid Figure 1. Transition temperatures of liquid crystal N-(4-methoxybenzylidene)-4-butylaniline (MBBA). As suggested by the name, liquid crystals combine some properties of conventional liquids with those of crystals. Molecules in the liquid state are randomly oriented, whereas molecules in crystalline phases are arranged in a lattice with orientational order. Liquid crystals can be defined S3 as "ordered fluids" because their molecules have some elements of orientational and/or positional order. Hence, they exhibit some properties characteristic of the crystalline state, but the molecules are free to diffuse and flow (Fig. 2).2 Figure 2. Transitions between solid, liquid crystalline and liquid states of marter Additional chiral dopant Figure 3. Cholesteric liquid crystal obtained by addition of a chiral molecule to an achiral nematic host: p is the pitch of the helix MBBA EBBA Chiral dopant Chart 1. The chiral dopant N-(4-phenylbenzylidene)-1-phenylethanamine will be mixed with the mixture of two nematic liquid crystals N-(4-methoxybenzylidene)-4-butylaniline (MBBA) and 4- (ethoxybenzylidene)-4-butylanline (EBBA). Cholesteric LCs can be obtained by dissolving a chiral compound, the "dopant", in an achiral nematic LC host. Addition of the chiral dopant induces the helical structure of the chiral nematic liquid crystalline phase, defined by its pitch p, where p is the length of the helical axis over a full 360 rotation of the rod-like molecules.3 As helices are inherently chiral, the configuration of the constituent molecules is manifested in the structure of the bulk phase. The two enantiomers of a chiral compound give helices that twist in opposite directions (ie. left- or right-handed helices). The compounds to be used in this experiment are given in Chart 1 A useful property of cholesteric phases is the selective reflection of polarized light (not the same as the optical rotation of linearly polarized light taught in lecture). Incident light with a A useful property of cholesteric phases is the selective reflection of polarized light (not the same as the optical rotation of linearly polarized light taught in lecture). Incident light with a S4 wavelength similar to the pitch (p) of the helix is reflected when it strikes a film of a cholesteric liquid crystal. The observed color therefore depends on the pitch of the helix. The reflected light has another important property: it is circularly polarized, with the polarization dependent on the twist sense of the cholesteric helix. The material reflects only circular polarized light with the same handedness as the handedness of the helix. The opposite handedness is transmitted through the material. In other words, a left-handed helix will selectively reflect left-handed circularly polarized light. This combination of properties has found broad application in the production of liquid crystal thermometers. This method can also be used for enantiomeric excess (ee) determination by visual color inspection. The method makes use of the sensitivity of the pitch towards the strength of the chiral perturbation. A chiral analyte of interest is doped into the liquid crystal, and, based on its enantiomeric excess, a whole spectrum of different colors can be obtained, allowing the ee to be rapidly estimated Incident light Reflected light Figure 4. Selective reflection of the circularly polarized light Chirality plays a very important role in the pharmaceutical industry. For example, amino acids are the building blocks of proteins and enzymes in our body. All standard amino acids, except glycine, are chiral. Therefore, the chemistry of our bodies is controlled by chiral molecules. This plays a very important role in drug design and development as proteins are often selective towards one stereoisomer. Although one enantiomer of the drug may be therapeutic, the other can cause irreparable harm. The drug thalidomide is a perfect example of how chirality impacts differing selectivity. It was designed as a treatment for morning sickness for pregnant women (Chart 2). Although enantiomerically pure (R)-thalidomide possesses a sedative effect and helps calm down the nervousness of pregnant women who ingest it. (S)-thalidomide causes a specific birth defect known as phocomelia (deformed limbs). Presently, the government closely monitors chirality in drug development to minimize side effects . 3.8C sickness for pregnant women (Chart 2). Although enantiomerically pure (R)-thalidomide possesses a sedative effect and helps calm down the nervousness of pregnant women who ingest it, (S)-thalidomide causes a specific birth defect known as phocomelia (deformed limbs). Presently, the government closely monitors chirality in drug development to minimize side effects. o ofte gore -NH 0 Chart 2. (R)-Thalidomide (left) and (S)-thalidomide (right) In the experiment that follows, you will observe the interaction between chiral dopants and nematic liquid crystalline hosts as indicated by light reflection. The absolute stereochemistry of the dopant will be also determined. Part 1: Synthesis of the Chiral Dopant NH2 NH2 Chart 3. Starting materials to synthesize the chiral dopant: (R)-1-phenylethylamine (left). (S)- 1-phenylethylamine (center), biphenyl-4-carboxaldehyde (right) Protocol: 1. Obtain 0.26 mL of 1-phenylethylamine of unknown configuration (your instructor will give you the sample) 2. Weigh 0.25 g of biphenyl-4-carboxaldehyde in a weigh boat. 3. Measure 6.0 mL 95% ethanol. 4. Transfer all materials to 50 mL round bottom flask, which will be the reflux flask. Make sure to transfer all the amine over by rinsing vial with ethanol. Protocol: 1. Obtain 0.26 mL of 1-phenylethylamine of unknown configuration. (your instructor will give you the sample) 2. Weigh 0.25 g of biphenyl-4-carboxaldehyde in a weigh boat. 3. Measure 6.0 mL 95% ethanol. 4. Transfer all materials to 50 mL round bottom flask, which will be the reflux flask. Make sure to transfer all the amine over by rinsing vial with ethanol. 5. Add stir bar to reflux flask and set up reflux apparatus. 6. Heat at reflux for 15 minutes once the mixture starts boiling. 7. Measure about 7 mL of 95% ethanol into a small graduated cylinder and place graduated cylinder in beaker filled with ice. 8. After 15 minutes of boiling, take heat off and allow the solution to cool to room temperature. 9. Place the cooled flask into beaker filled with ice. 10. Collect crystals by vacuum filtration through a Buchner funnel. 11. Wash crystals with no more than 7 mL total of ice cold ethanol. 12. Leave on Buchner funnel, when crystals are completely dried, collect them into a previously tared weigh boat and determine mass of sample. 13. Obtain melting point and IR spectrum. Part 2: Mixing LCs with Dopant Prepare a 13 % by mass mixture of synthesized chiral dopant and liquid crystals 1. Weigh 0.10 0.01 g of EBBA and measure 0.10 + 0.01 mL of MBBA. 2. Weigh 0.030 + 0.003 g of synthesized chiral dopant. (It is very important to keep the liquid crystal in a 1:1 ratio) 3. Measure the melting point and the clearing temp of EBBA. 4. Vigorously grind these substances in a mortar with a pestle for about 3 minutes until you get a slightly opalescent mixture. Let it sit for 2 more minutes. 5. Place a drop of the LC-chiral dopant mixture on a glass slide and cover with cover slip. Characterization 1. Using the LC/chiral dopant mixture prepared earlier, shear the cover slip on your mixture. 2. Observe what happens while shearing the cover slip, alternating between different lenses of the 3D glasses by covering one lens at a time with your palm. 3. Record your observations. 4. Slightly heat your glass slides. Once the mixture on the glass slide is no longer in the liquid crystal phase, shear the cover slip. Observe what happens while shearing cover slips again alternating between different lenses of the 3D glasses. Record your observations 5. Let your glass slides cool down to room temperature. Observe your glass slides with and without shearing of the cover slips, alternating between different lenses of the 3D glasses, Characterization 1. Using the LC/chiral dopant mixture prepared earlier, shear the cover slip on your mixture. 2. Observe what happens while shearing the cover slip, alternating between different lenses of the 3D glasses by covering one lens at a time with your palm. 3. Record your observations. 4. Slightly heat your glass slides. Once the mixture on the glass slide is no longer in the liquid crystal phase, shear the cover slip. Observe what happens while shearing cover slips again alternating between different lenses of the 3D glasses. Record your observations. 5. Let your glass slides cool down to room temperature. Observe your glass slides with and without shearing of the cover slips, alternating between different lenses of the 3D glasses. Record your observations. 6. Obtain two glass slides from your instructor, one with known (R)-dopant in the mixture and one with the (S)-dopant. Shear the glass slides in the same way as you did for the glass slides with your LC/dopant mixtures and with the racemic mixture. Record your observations for each lens of the 3D glasses, 7. Now with each lens calibrated, determine which enantiomer you were assigned 8. Find a classmate who has the enantiomer of your compound and make a 13% racemic dopant-liquid crystals mixture using both of your samples. Place a drop of this mixture on a glass slide. Cover with a cover slip. Shear, heat, and cool down the glass slide in the same way as you did for the glass slides with your LC/chiral dopant mixtures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


