Question: = 1. (a) From two relationships, dAG mix = AG dx + AG2dX2 AG mix = AG X1 + AG2X2 determine the relationship between partial
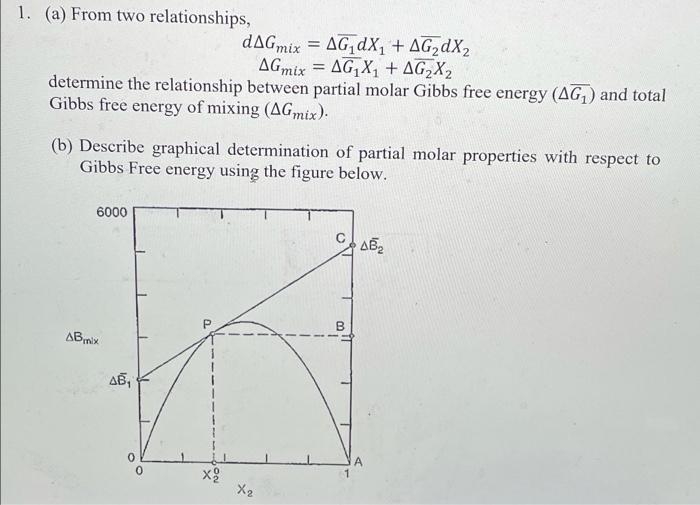
= 1. (a) From two relationships, dAG mix = AG dx + AG2dX2 AG mix = AG X1 + AG2X2 determine the relationship between partial molar Gibbs free energy (AG) and total Gibbs free energy of mixing (AGmix). (b) Describe graphical determination of partial molar properties with respect to Gibbs Free energy using the figure below. 6000 AB, B 1 mix , A x2 X2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


