Question: 1. A seven-component mixture is flashed at a fixed P and T. a) Using the K values and feed composition below, make a plot of
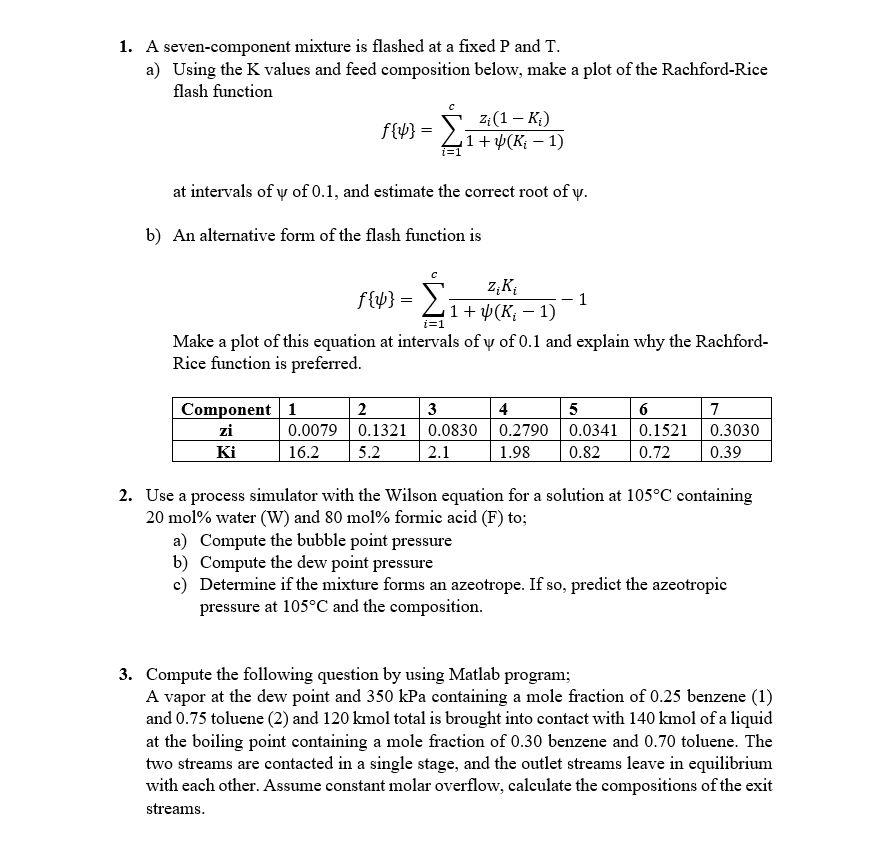
1. A seven-component mixture is flashed at a fixed P and T. a) Using the K values and feed composition below, make a plot of the Rachford-Rice flash function f{}=i=1c1+(Ki1)zi(1Ki) at intervals of of 0.1 , and estimate the correct root of . b) An alternative form of the flash function is f{}=i=1c1+(Ki1)ziKi1 Make a plot of this equation at intervals of of 0.1 and explain why the RachfordRice function is preferred. 2. Use a process simulator with the Wilson equation for a solution at 105C containing 20mol% water (W) and 80mol% formic acid (F) to; a) Compute the bubble point pressure b) Compute the dew point pressure c) Determine if the mixture forms an azeotrope. If so, predict the azeotropic pressure at 105C and the composition. 3. Compute the following question by using Matlab program; A vapor at the dew point and 350kPa containing a mole fraction of 0.25 benzene (1) and 0.75 toluene (2) and 120kmol total is brought into contact with 140kmol of a liquid at the boiling point containing a mole fraction of 0.30 benzene and 0.70 toluene. The two streams are contacted in a single stage, and the outlet streams leave in equilibrium with each other. Assume constant molar overflow, calculate the compositions of the exit streams
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


