Question: 1. (a) Write out all applicable aluminum complex formation and equilibrium relationship equations, including log K values. (b) Solve for log concentration values in terms

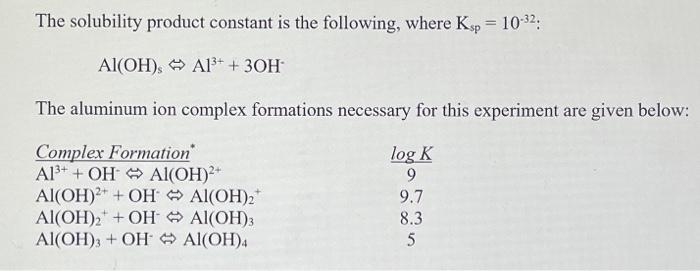
1. (a) Write out all applicable aluminum complex formation and equilibrium relationship equations, including log K values. (b) Solve for log concentration values in terms of pH (i.c. log [AP], etc.). The solubility product constant is the following, where Ksp = 10-32: Al(OH), A13+ + 30H The aluminum ion complex formations necessary for this experiment are given below: Complex Formation A13+ + OH AlOH)2 Al(OH)2+ + OH Al(OH)2 Al(OH)2 + OH Al(OH)3 Al(OH)3 + OH Al(OH)4 log K 9 9.7 8.3 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


