Question: 1. Assuming standard conditions for all reactants and products, write the relevant half-cell reactions, calculate the cell potential (voltage, use EMF Table 2.1 on following

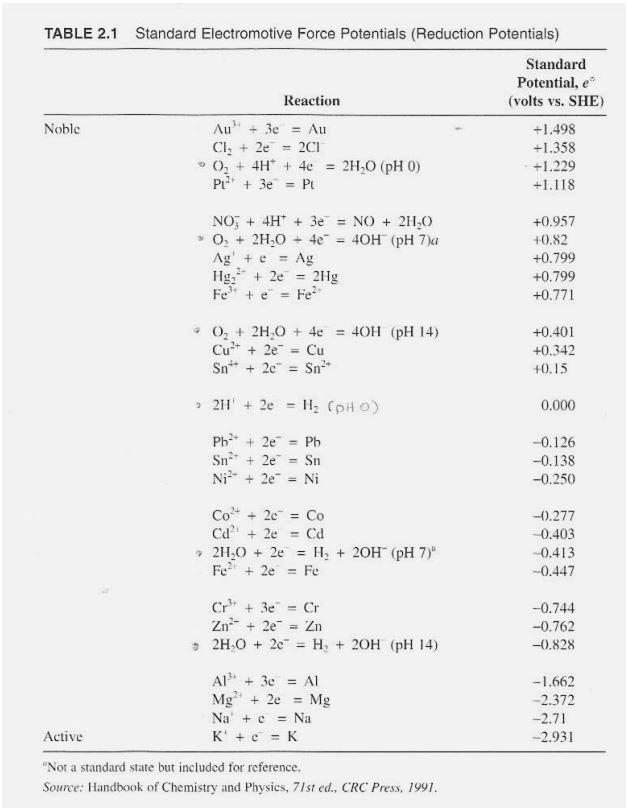
1. Assuming standard conditions for all reactants and products, write the relevant half-cell reactions, calculate the cell potential (voltage, use EMF Table 2.1 on following page), and determine the spontaneous reaction direction for the following: a. Fe2+ + H2 = Fe + 2H+ b. 3ZnSO4 + 2Al = 3Zn + Al2(SO4)3 TABLE 2.1 Standard Electromotive Force Potentials (Reduction Potentials) Standard Potential, e Reaction (volts vs. SHE) Noble Au + 3 = Au +1.498 CI, + 2e 2C1 +1.358 02 + 4H* + 4e 2H.0 (pH 0) +1.229 PL? + 3e = PL +1.118 ti = NO; + 4H+ + 3e = NO + 2H20 0, + 2H20 + 4e = 40H (pH 7)a Ag' + e Ag Hg. + 2e = 2Hg Fete Fe +0.957 +0.82 +0.799 +0.799 +0.771 0, + 2H,0 + 40 = 40H (pH 14) Cu?+ + 2e = Cu Sn- + 2e Sn +0.401 +0.342 +0.15 211 + 2e , ) 0.000 Pb2+ + 2e = Pb Snat + 2e Sn Ni2- + 2e Ni -0.126 -0.138 -0.250 Co2+ + 20 = Co Ca + 2e = Cd 24,0 + 2e = H2 + 20H (pH 7)" + 2e = Fe -0.277 0.403 -0.413 -0.447 - Cr + 3e Cr Zn + 2e Zn 52H,0 + 20 = H, + 2OH (PH 14) -0.744 -0.762 -0.828 Al' + 3 = Al Mg? + 2e Mg Na + c = Na K' + c = K -1.662 -2.372 -2.71 - 2.931 Active "Not a standard state but included for reference. Source: Handbook of Chemistry and Physics, 71st ed.. CRC Press, 1991
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


