Question: Page 3 - Measuring Cell Potentials Pre-Lab Questions Standard reduction potentials (Ed.) are defined for reduction hall-reactions. For a voltaic cell, Ere for the cathode

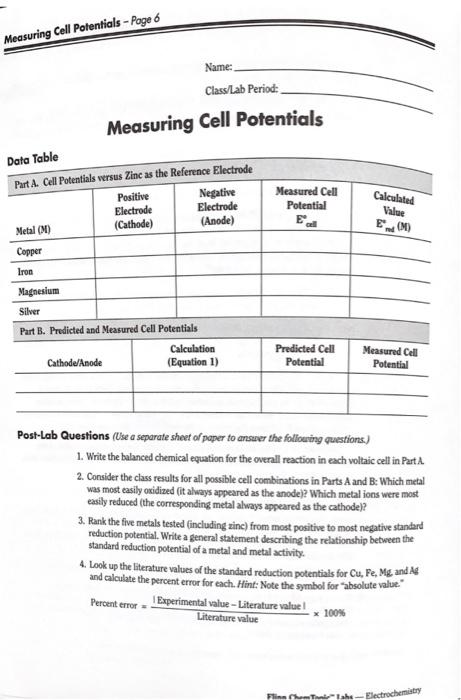

Page 3 - Measuring Cell Potentials Pre-Lab Questions Standard reduction potentials (Ed.) are defined for reduction hall-reactions. For a voltaic cell, Ere for the cathode (reduction) must be more positive than for the anode (oxida- tion). Consider the following two metals and their standard reduction potentials. Com"(aq) + 2 Cols) - -0.28V Aglag) + e - Agls) - 0.80 V 1. If half-cells for these two metals were combined in a voltaic cell, what reaction would take place at the anode? What reaction would take place at the cathode? 2. Calculate the cell potential for the overall (spontaneous) reaction under standard conditions. 3. Write the balanced chemical equation for the overall reaction. 4. The activity of a metal is defined as its ease of oxidation (an active metal is one that is easily oxidized). Based on their standard reduction potentials, which is the more active metal, cobalt or silver? Materials Copper strips or foil. Cu, 1-cm? Beral-type pipets or eyedroppers, 6 Copper(II) sulfate solution, CuSO, 1 M, 1 mL Computer interface system (LabPro) ron strips or sheet, Fe, 1-cm? Data collection software (LoggerPro) Vron(II) sulfate solution, Feso, 1 M, 1 ml Filter paper, quantitative, 9-cm Magnesium ribbon, Mg, 1 cm Multimeter or Voltage probe Magnesium sulfate solution, MgO, 1 M, 1 mL Petri dish or acetate transparency Silver foil, Ag. 1-cm? Sandpaper or steel wool Silver nitrate solution, AgNO, 1 M, 1 mL Scissors Sodium nitrate solution, NaNO, 1 M, 2 mL Tweezers or forceps Zinc strips or foil, Zn, 1-cm Wash bottle and distilled water Zinc sulfate solution, Zno, 1 M, 1 mL White paper and pencil Safety Precautions Silver nitrate solution is a corrosive liquid and toxic by ingestion. It will stain skin and clothing. Copper(II) sulfate solution is toxic by ingestion. Iran(II) sulfate and zinc sulfate solutions are slightly toxic. Magnesium metal is a flammable solid; avoid contact with Names and heat. Metal pieces may have sharp edges handle with care. Avoid contact of all chemicals with eyes and skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Wash hands thoroughly with soup and water before leap ing the lab. Measuring Cell Potentials Measuring Cell Potentials - Page 6 Name: Class/Lab Period: Measuring Cell Potentials Data Table Part A. Cell Potentials versus Zinc as the Reference Electrode Positive Negative Electrode Electrode Measured Cell Potential E all Metal (M) (Cathode) (Anode) Copper Iron Magnesium Silver Part B. Predicted and Measured Cell Potentials Calculation Cathode/Anode (Equation 1) Predicted Cell Potential Post-Lab Questions (Use a separate sheet of paper to answer the following questions.) Calculated Value End (M) Measured Cell Potential 1. Write the balanced chemical equation for the overall reaction in each voltaic cell in Part A 2. Consider the class results for all possible cell combinations in Parts A and B: Which metal was most easily oxidized (it always appeared as the anode)? Which metal ions were most easily reduced (the corresponding metal always appeared as the cathode)? 3. Rank the five metals tested (including zinc) from most positive to most negative standard reduction potential. Write a general statement describing the relationship between the standard reduction potential of a metal and metal activity. 4. Look up the literature values of the standard reduction potentials for Cu, Fe, Mg and Ag and calculate the percent error for each. Hint: Note the symbol for "absolute value. Percent error Experimental value-Literature value! Literature value x 100% Electrochemistry Pre-Lab Questions Standard reduction potentials (Ered) are defined for reduction half-reactions. For a voltaic cell, Ered for the cathode (reduction) must be more positive than Ered for the anode (oxida- tion). Consider the following two metals and their standard reduction potentials. Co+ (aq) + 2e Co(s) E = -0.28 V red Ag (aq) + e Ag(s) E red=0.80 V 1. If half-cells for these two metals were combined in a voltaic cell, what reaction would take place at the anode? What reaction would take place at the cathode? 2. Calculate the cell potential for the overall (spontaneous) reaction under standard conditions. 3. Write the balanced chemical equation for the overall reaction. 4. The activity of a metal is defined as its ease of oxidation (an active metal is one that is easily oxidized). Based on their standard reduction potentials, which is the more active metal, cobalt or silver? Copper strips or foil, Cu, 1-cm Copper(II) sulfate solution, CuSO,, 1 M, 1 mL Iron strips or sheet, Fe, 1-cm Beral-type pipets or eyedroppers, 6 Computer interface system (LabPro) Data collection software (LoggerPro) Filter paper, quantitative, 9-cm Multimeter or Voltage probe Iron(II) sulfate solution, FeSO,, 1 M, 1 mL Magnesium ribbon, Mg, 1 cm Petri dish or acetate transparency Magnesium sulfate solution, MgSO, 1 M, 1 mL Silver foil, Ag, 1-cm Sandpaper or steel wool Scissors Silver nitrate solution, AgNO3, 1 M, 1 mL Sodium nitrate solution, NaNO3, 1 M, 2 mL Zinc strips or foil, Zn, 1-cm- Tweezers or forceps Wash bottle and distilled water White paper and pencil Zinc sulfate solution, ZnSO4, 1 M, 1 mL Silver nitrate solution is a corrosive liquid and toxic by ingestion. It will stain skin and clothing. Copper(II) sulfate solution is toxic by ingestion. Iron(II) sulfate and zinc sulfate solutions are slightly toxic. Magnesium metal is a flammable solid; avoid contact with flames and heat. Metal pieces may have sharp edges-handle with care. Avoid contact of all chemicals with eyes and skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Wash hands thoroughly with soap and water before leav- ing the lab. Materials Safety Precautions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


