Question: 1. Assuming the approximate masses given below for the stock solutions are exact, calculate the concentration of each analyte in each standard solution you will
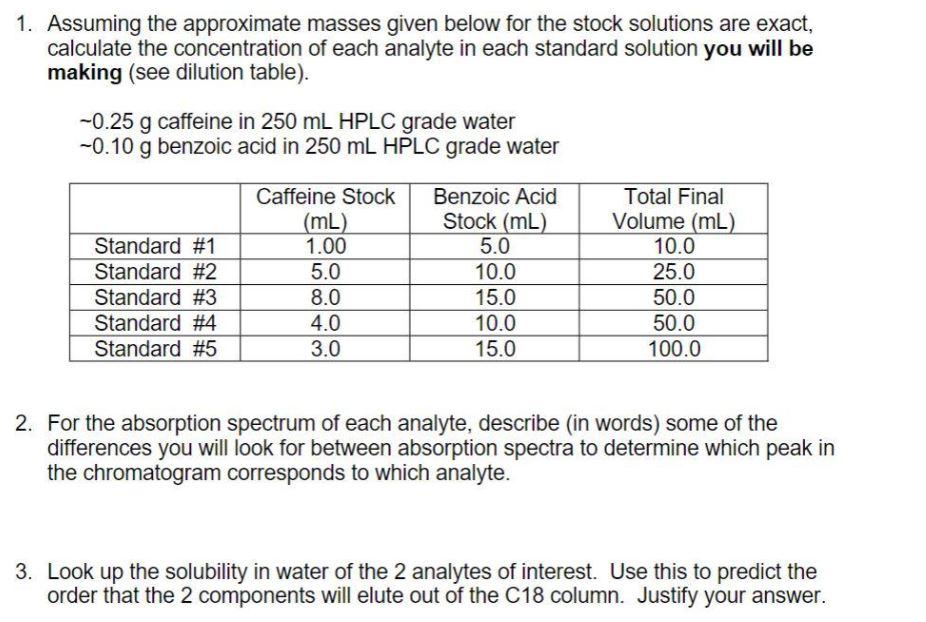
1. Assuming the approximate masses given below for the stock solutions are exact, calculate the concentration of each analyte in each standard solution you will be making (see dilution table). -0.25 g caffeine in 250 mL HPLC grade water -0.10 g benzoic acid in 250 mL HPLC grade water Standard #1 Standard #2 Standard #3 Standard #4 Standard #5 Caffeine Stock (mL) 1.00 5.0 8.0 4.0 3.0 Benzoic Acid Stock (mL) 5.0 10.0 15.0 10.0 15.0 Total Final Volume (mL) 10.0 25.0 50.0 50.0 100.0 2. For the absorption spectrum of each analyte, describe (in words) some of the differences you will look for between absorption spectra to determine which peak in the chromatogram corresponds to which analyte. 3. Look up the solubility in water of the 2 analytes of interest. Use this to predict the order that the 2 components will elute out of the C18 column. Justify your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


