Question: 1. Before you start the Grignard reaction you will need to exchange your glassware (cleaned) for dry glassware from the oven. Why does your glassware
1. Before you start the Grignard reaction you will need to exchange your glassware (cleaned) for dry glassware from the oven. Why does your glassware need to be dry for this reaction?
a Moisture reacts with the Grignard reagent producing benzene and magnesium hydroxide.
b Moisture reacts with the magnesium metal and produces hydrogen gas and magnesium hydroxide.
c Moisture reacts with the bromobenzene producing phenol and hydrogen chloride gas.
d Moisture reacts with the magnesium metal producing magnesium (II) hydrate.
e Moisture reacts with the Grignard reagent producing phenol and magnesium hydride.
f Moisture reacts with bromobenzene to produce benzene and hypobromous acid (HOBr).
2. As you are adding benzaldehyde to the Grignard reagent you will see a violent reaction and the formation of a solid. Select the structure that corresponds to the solid seen at this point in the reaction.
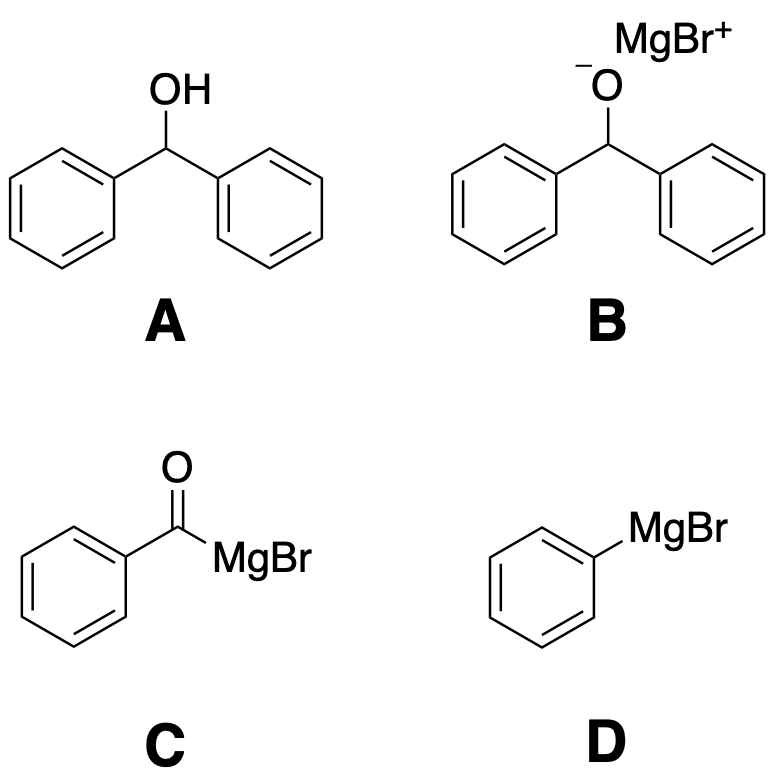
MgBr+ OH A MgBr MgBr D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


