Question: 1) First read the experiment introduction and the procedures then could you fill out the reagent table MW, Grams, Moles and theoretical yield of Triphenylmethanol

1) First read the experiment introduction and the procedures then could you fill out the reagent table MW, Grams, Moles and theoretical yield of Triphenylmethanol in moles and in grams?
2) What is the minimum amount of water (in mls) needed to quench the Grignard reagent in the above experiment? (NB This is a calculation)?
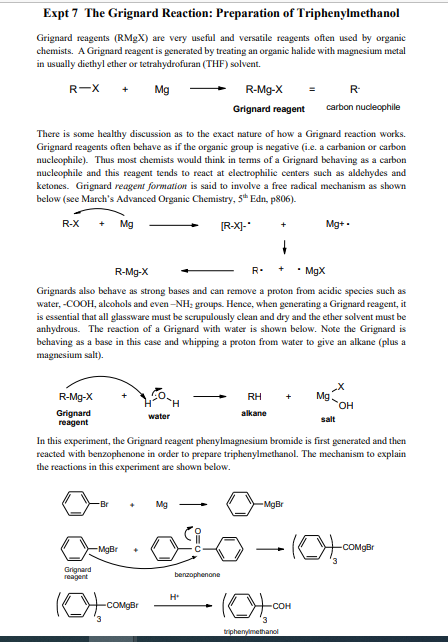
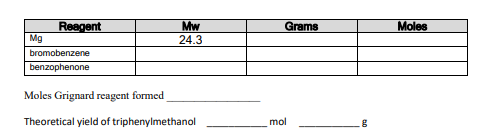
Expt 7 The Grignard Reaction: Preparation of Triphenylmethanol Grignard reagents (RMX) are very useful and versatile reagents often used by organic chemists. A Grignard reagent is generated by treating an organic halide with magnesium metal in usually diethyl ether or tetrahydrofuran (THF) solvent. R-X + Mg R-Mg-X R Grignard reagent carbon nucleophile There is some healthy discussion as to the exact nature of how a Grignard reaction works. Grignard reagents often behave as if the organic group is negative (i.e. a carbanion or carbon nucleophile). Thus most chemists would think in terms of a Grignard behaving as a carbon nucleophile and this reagent tends to react at electrophilie centers such as aldehydes and ketones. Grignard reagent formation is said to involve a free radical mechanism as shown below (see March's Advanced Organic Chemistry, 5th Edn, p&06). R-X Mg [R-X]- Mg+. R-Mg-X R. MgX Grignards also behave as strong bases and can remove a proton from acidic species such as water, -COOH, alcohols and even-NH: groups. Hence, when generating a Grignard reagent, it is essential that all glassware must be scrupulously clean and dry and the ether solvent must be anhydrous. The reaction of a Grignard with water is shown below. Note the Grignard is behaving as a base in this case and whipping a proton from water to give an alkane (plus a magnesium salt). R-Mg-X RH Mg H H Grignard water alkane reagent salt In this experiment, the Grignard reagent phenylmagnesium bromide is first generated and then reacted with benzophenone in order to prepare triphenylmethanol. The mechanism to explain the reactions in this experiment are shown below. Mg -MgBr MgBr -lot , COMBI Grignard reagent benzophenone H (C), COMgBr lot.com triphenyimethanol Procedure Make sure all glassware is dry before commencing this experiment. Assemble a clean dry 250 or 500ml RBF containing magnesium (Ig. 41 mmol) turnings with a condenser and CaCl2 tube on top. Add sodium-dried diethyl ether (6ml) to the RBF followed by a crystal of iodine. Dissolve bromobenzene (19 mmol, 3g, 2 ml) in 4 ml sodium dried ether and add to the addition funnel. Add dropwise with stirring and watch the Grignard reagent form. Usually, once the Grignard reaction has started, the contents in the addition funnel can be diluted with additional ether (5ml) in order to control the exotherm. If no reaction has begun after 5 min, warm the reaction pot slightly in a beaker of hot water. As the reaction subsides and the Grignard solution is cooling to room temperature, prepare the benzophenone (Ilmmol, 2g) in sodium-dried ether (6 ml) and add to the addition funnel. Cool the reaction flask using an ice bath (in order to control the subsequent exothermic reaction of Grignard and ketone) and then add the benzophenone solution dropwise. Observe the reaction flask for signs that a reaction is taking place. Once addition is complete, remove the ice-bath and allow the stirring reaction mixture warm to room temperature. Product formation should be apparent at this stage. If no crystallization is noted, warm the reaction flask in a hot water bath for 5 min. Add 5% H2SO4 (aq) (5ml) to the stirring contents of the reaction flask. If stirring is not possible, swirl the flask manually in the hood as the aq. acid is added. This step quenches any unreacted Grignard reagent plus protonates the alkoxide intermediate to give the triphenylmethanol product. The magnesium salts become soluble in the aqueous medium and the triphenylmethanol product dissolves in the ether layer. Transfer to a separatory funnel leaving behind any undissolved solids. Rinse the flask with ether (-5ml) and add to the separatory funnel. Shake the funnel and allow the layers to separate. Drain off the lower aqueous layer and keep it aside. Wash the ether layer with 5% H2SO4 (aq) (3ml), drain off the lower layer. Then wash with satd NaCl (aq) (3ml) and drain off. Dry the ether layer over anhyd Na2SO4. Decant the dried ether phase into a clean dry pre-weighed RBF and remove the ether on the rotary evaporator. Weigh the crude product. Calculate a % yield and keep a tiny amount aside for a mp. The crude contains triphenylmethanol product, unreacted starting materials and a main by-product, biphenyl. Recrystallize from hexanes and determine % recovery, IR and mp (literature mp is -162C). Grams Moles Mw 24.3 Reagent Mg bromobenzene benzophenone Moles Grignard reagent formed Theoretical yield of triphenylmethanol mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


