Question: 1. Calculate E (standard cell potential), E (cell potential), and AG (free energy change) for the following cell reaction. Would the following reaction proceed
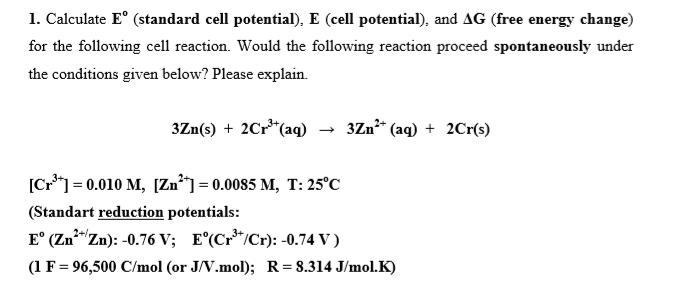
1. Calculate E (standard cell potential), E (cell potential), and AG (free energy change) for the following cell reaction. Would the following reaction proceed spontaneously under the conditions given below? Please explain. 3Zn(s) + 2Cr*(aq) - 3Zn* (aq) + 2Cr(s) [Cr*] = 0.010 M, [Zn] = 0.0085 M, T: 25C (Standart reduction potentials: E (Zn*"Zn): -0.76 V; E(Cr*/Cr): -0.74 V) 2+/. (1 F= 96,500 C/mol (or J/V.mol); R= 8.314 J/mol.K)
Step by Step Solution
3.31 Rating (172 Votes )
There are 3 Steps involved in it
To solve this problem we need to calculate the standard cell potential Ecirc the cell potential E an... View full answer

Get step-by-step solutions from verified subject matter experts


