Question: 2. a. Calculate the electromotive force, or emf (E) for the following concentration cell at 25C: Cu(s) | Cu* (aq, 0.080 M) | (KCI,
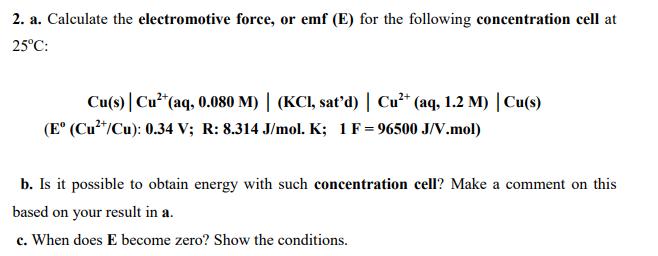
2. a. Calculate the electromotive force, or emf (E) for the following concentration cell at 25C: Cu(s) | Cu* (aq, 0.080 M) | (KCI, sat'd) | Cu* (aq, 1.2 M) | Cu(s) (E (Cu"/Cu): 0.34 V; R: 8.314 J/mol. K; 1F= 96500 J/V.mol) b. Is it possible to obtain energy with such concentration cell? Make a comment on this based on your result in a. c. When does E become zero? Show the conditions.
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
To solve the problem lets break it down into parts a Calculate the electromotive force emf E for the ... View full answer

Get step-by-step solutions from verified subject matter experts


