1. Calculate the density of O2, in g/L, at STP. 1.62 g/L b. 0,714 g/L 1.43...
Fantastic news! We've Found the answer you've been seeking!
Question:
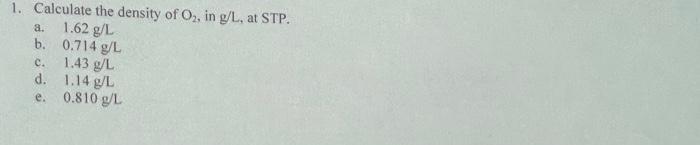
Related Book For 

Posted Date:




