Question: 1. Classify the following solids as either: A. Molecular solid B. Metallic solid C. Ionic solid D. Network covalent solid For your answer insert one

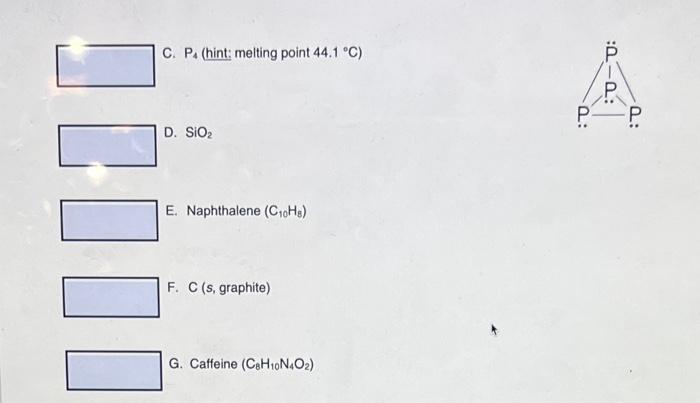
1. Classify the following solids as either: A. Molecular solid B. Metallic solid C. Ionic solid D. Network covalent solid For your answer insert one of the choices A - D in the answer box. Example, if the answer is metallic, then the answer is choice B. A. Ca3(PO4)2 B. Fe 1 C. P4 (hint: melting point 44.1C ) D. SiO2 E. Naphthalene (C10H8) F. C (s, graphite) G. Caffeine (C8H10N4O2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


