Question: 1. Consider a gas phase reaction (methanation reaction) given by: A + 3B + C + D Assume that the mixture behaves as an ideal
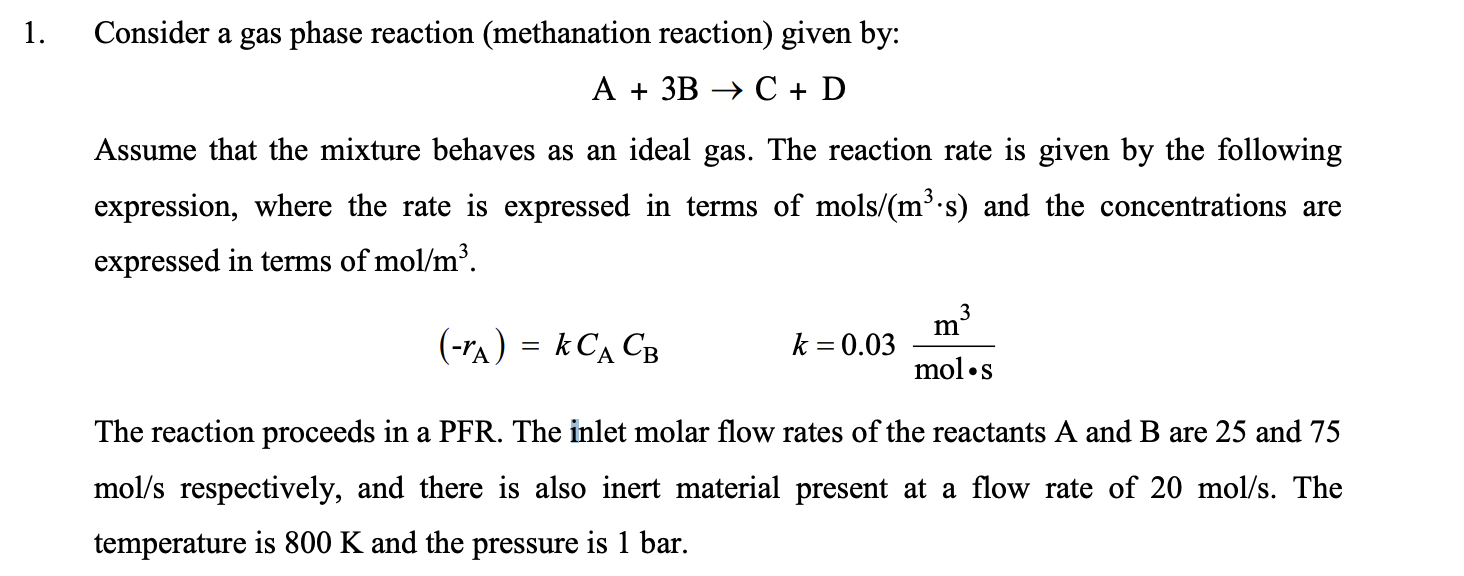

1. Consider a gas phase reaction (methanation reaction) given by: A + 3B + C + D Assume that the mixture behaves as an ideal gas. The reaction rate is given by the following expression, where the rate is expressed in terms of mols/(m.s) and the concentrations are expressed in terms of mol/m?. 3 m (-A) = k CACB = k=0.03 mols The reaction proceeds in a PFR. The inlet molar flow rates of the reactants A and B are 25 and 75 mol/s respectively, and there is also inert material present at a flow rate of 20 mol/s. The temperature is 800 K and the pressure is 1 bar. 2. Consider the same reaction that occurs in Problem 1. Now the reaction will occur in a perfectly mixed CSTR. (a) Compute the volume required to achieve 70 % conversion. (b) Compute the space time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


