Question: please help!2007 1. (30) The gas phase reaction 24+B2D can be described by the rate equation: r ( -1, = 5.0 C,C, (smo) liter min)
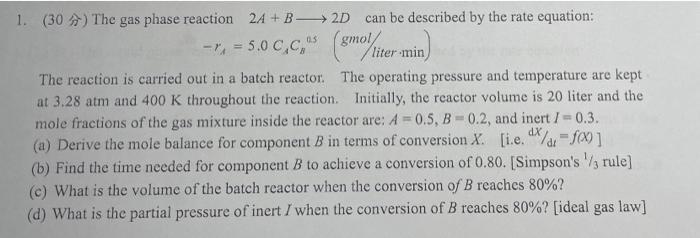
1. (30) The gas phase reaction 24+B2D can be described by the rate equation: r ( -1, = 5.0 C,C," (smo) liter min) The reaction is carried out in a batch reactor. The operating pressure and temperature are kept at 3.28 atm and 400 K throughout the reaction. Initially, the reactor volume is 20 liter and the mole fractions of the gas mixture inside the reactor are: A = 0.5, B -0.2, and inert / -0.3. (a) Derive the mole balance for component B in terms of conversion X. [i.e. dx/dr-f(x)] (b) Find the time needed for component B to achieve a conversion of 0.80. [Simpson's rule) (c) What is the volume of the batch reactor when the conversion of B reaches 80%? (d) What is the partial pressure of inert I when the conversion of B reaches 80%? [ideal gas law]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


