Question: 1. Consider a molecule that exists in equilibrium between State A and State B. At equilibrium the fraction of [B]eq/[A]eq=0.1. AB A. Suppose a population
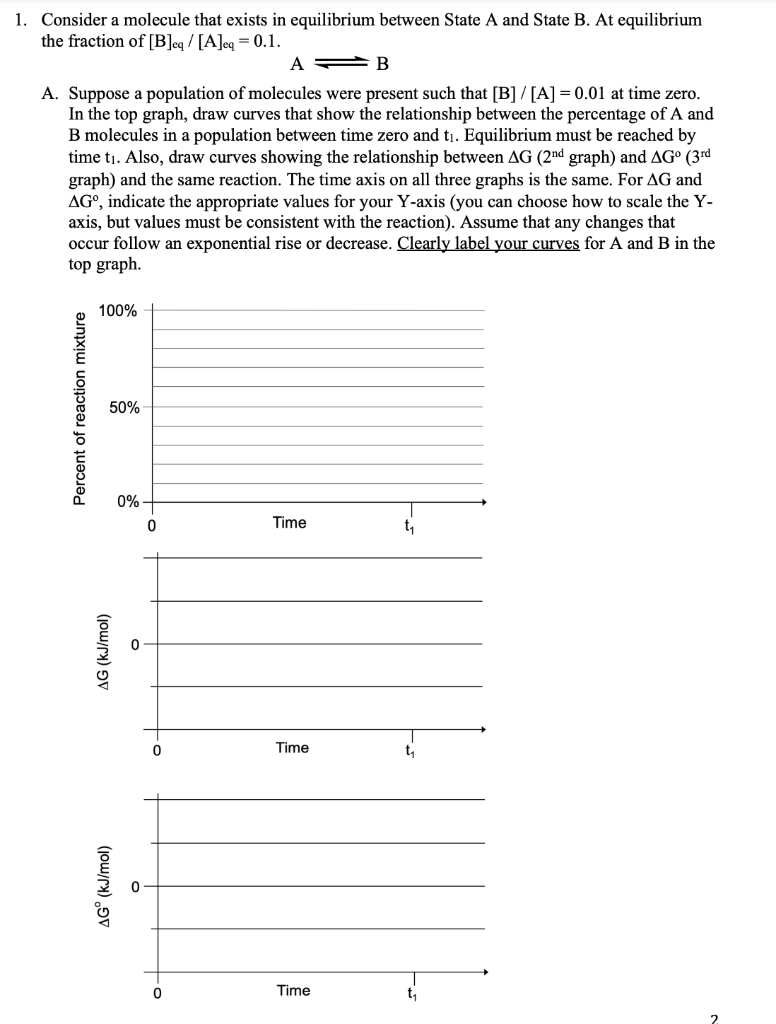
1. Consider a molecule that exists in equilibrium between State A and State B. At equilibrium the fraction of [B]eq/[A]eq=0.1. AB A. Suppose a population of molecules were present such that [B]/[A]=0.01 at time zero. In the top graph, draw curves that show the relationship between the percentage of A and B molecules in a population between time zero and t1. Equilibrium must be reached by time t1. Also, draw curves showing the relationship between G(2nd graph ) and G0(3rd graph) and the same reaction. The time axis on all three graphs is the same. For G and G, indicate the appropriate values for your Y-axis (you can choose how to scale the Y axis, but values must be consistent with the reaction). Assume that any changes that occur follow an exponential rise or decrease. Clearly label your curves for A and B in the top graph
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


