Question: 1. Consider the following phase diagram: MgO - Nio Data from TDnucl- Thermodata nuclear database T(K) 3200 3100 3000 2900 2800 2700 2600 2500
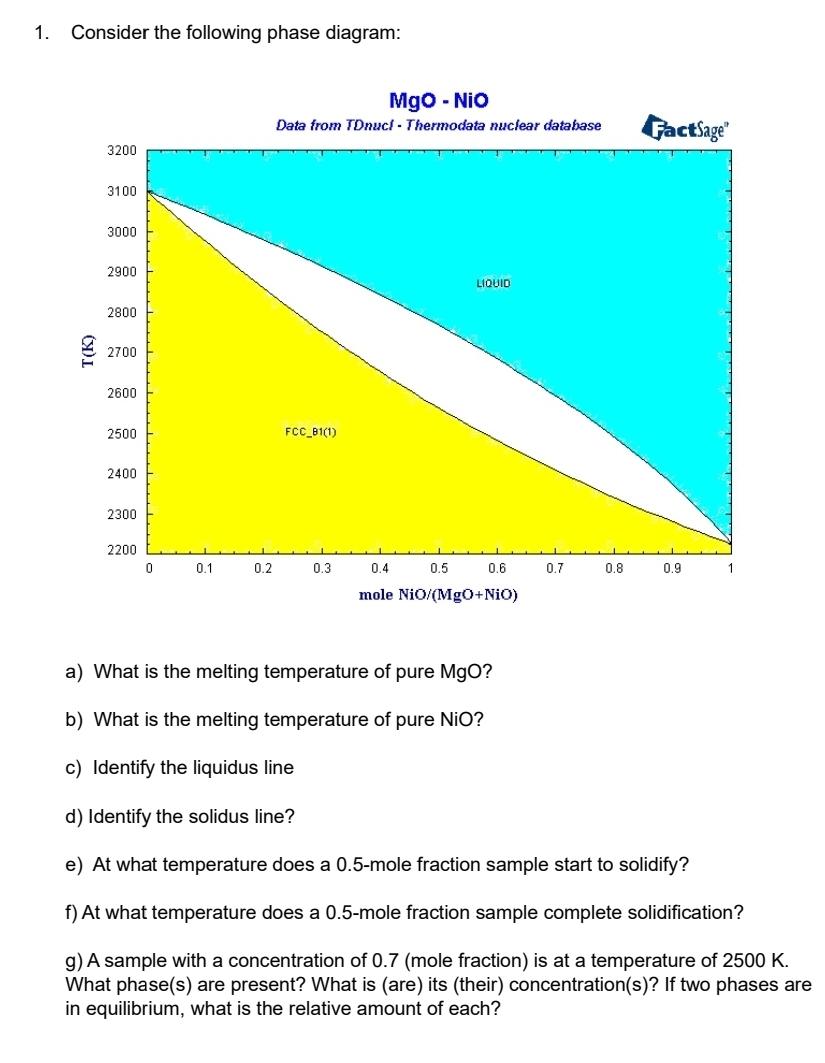
1. Consider the following phase diagram: MgO - Nio Data from TDnucl- Thermodata nuclear database T(K) 3200 3100 3000 2900 2800 2700 2600 2500 FCC_81(1) 2400 LIQUID FactSage 2300 2200 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 mole NiO/(MgO+NiO) a) What is the melting temperature of pure MgO? b) What is the melting temperature of pure NiO? c) Identify the liquidus line d) Identify the solidus line? e) At what temperature does a 0.5-mole fraction sample start to solidify? f) At what temperature does a 0.5-mole fraction sample complete solidification? g) A sample with a concentration of 0.7 (mole fraction) is at a temperature of 2500 K. What phase(s) are present? What is (are) its (their) concentration(s)? If two phases are in equilibrium, what is the relative amount of each?
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
a The melting temperature of pure MgO is the point where the solidus line intersects the MgO axis on ... View full answer

Get step-by-step solutions from verified subject matter experts


