Question: 1. Consider the structure below and answer the two questions: a. How many carbon atoms and hydrogens atoms are in the following structure? b. Convert
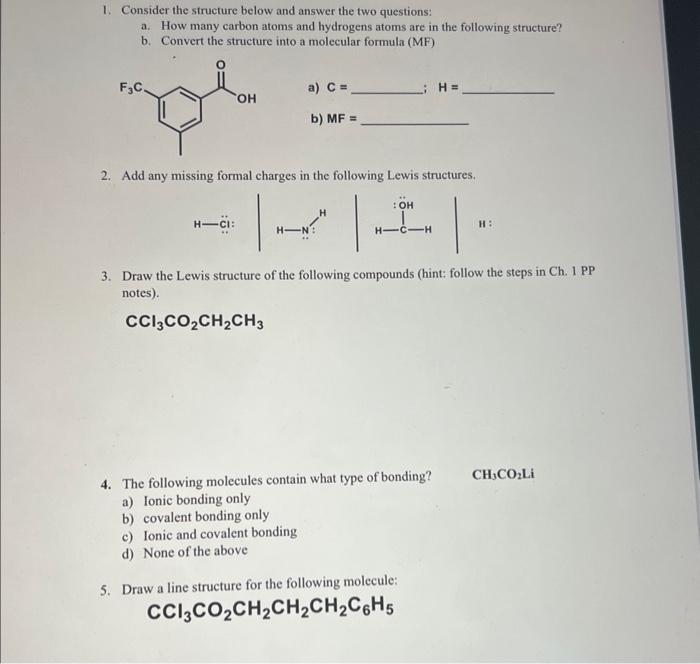
1. Consider the structure below and answer the two questions: a. How many carbon atoms and hydrogens atoms are in the following structure? b. Convert the structure into a molecular formula (MF) a) C= i. H= b) MF= 2. Add any missing formal charges in the following Lewis structures. 3. Draw the Lewis structure of the following compounds (hint: follow the steps in Ch. 1 PP notes). CCl3CO2CH2CH3 4. The following molecules contain what type of bonding? CH3CO2Li a) Ionic bonding only b) covalent bonding only c) Ionic and covalent bonding d) None of the above 5. Draw a line structure for the following molecule: CCl3CO2CH2CH2CH2C6H5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


