Question: 1. Consider the two molecules below. Propanoic acid Lactic acid 3 2 3 2. H C C-C H C C-C o: H. H :0.
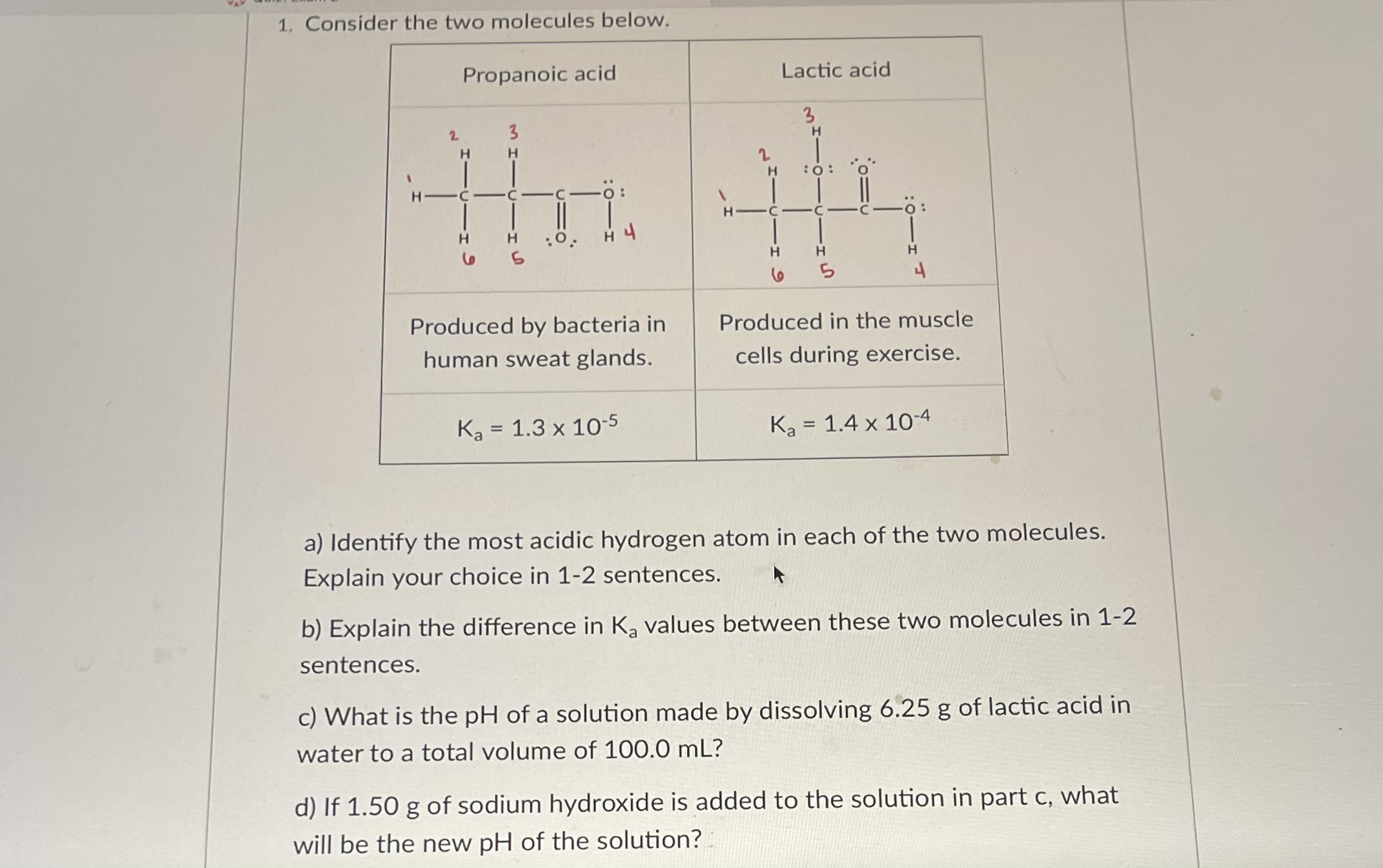
1. Consider the two molecules below. Propanoic acid Lactic acid 3 2 3 2. H C C-C H C C-C o: H. H :0. H 4 H. H. 5. Produced by bacteria in Produced in the muscle human sweat glands. cells during exercise. Ka = 1.3 x 10-5 Ka = 1.4 x 10-4 a) Identify the most acidic hydrogen atom in each of the two molecules. Explain your choice in 1-2 sentences. b) Explain the difference in K, values between these two molecules in 1-2 sentences. c) What is the pH of a solution made by dissolving 6.25 g of lactic acid in water to a total volume of 100.0 mL? d) If 1.50 g of sodium hydroxide is added to the solution in part c, what will be the new pH of the solution?
Step by Step Solution
3.49 Rating (142 Votes )
There are 3 Steps involved in it
A The most acidic Hydrogen atom in both the molecules in 4th H atom ... View full answer

Get step-by-step solutions from verified subject matter experts


