Question: 1) Consider this Equilibrium: 2 X(g) + 2 Y(g) + Zg) Keq = 6.5 x 10-7 @ 300 K If [X (g))} = 0.35 mol/L,
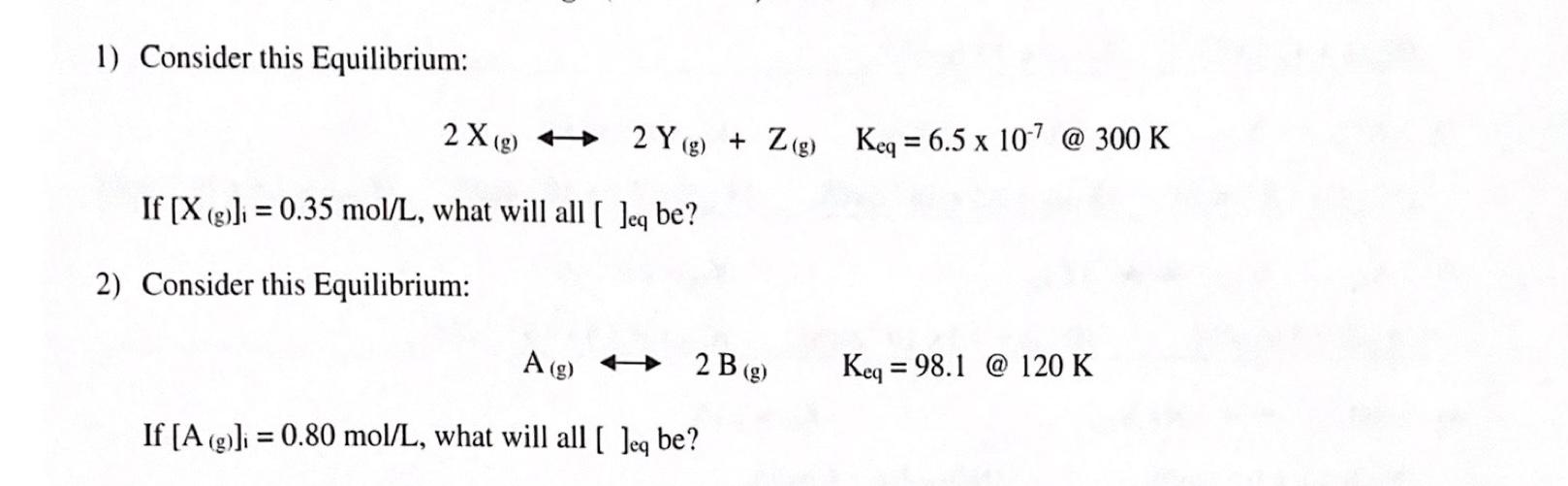
1) Consider this Equilibrium: 2 X(g) + 2 Y(g) + Zg) Keq = 6.5 x 10-7 @ 300 K If [X (g))} = 0.35 mol/L, what will all [ Jeg be? 2) Consider this Equilibrium: A(g) + 2 B (g) Keq = 98.1 @ 120 K = If [A (g))} = 0.80 mol/L, what will all ( Jeg be? =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


