Question: 1 . Determine a structure for the following compounds. Assign each peak and explain the coupling observed. ( The IR shows a carbonyl peak )
Determine a structure for the following compounds. Assign each peak and explain the coupling observed. The IR shows a carbonyl peak
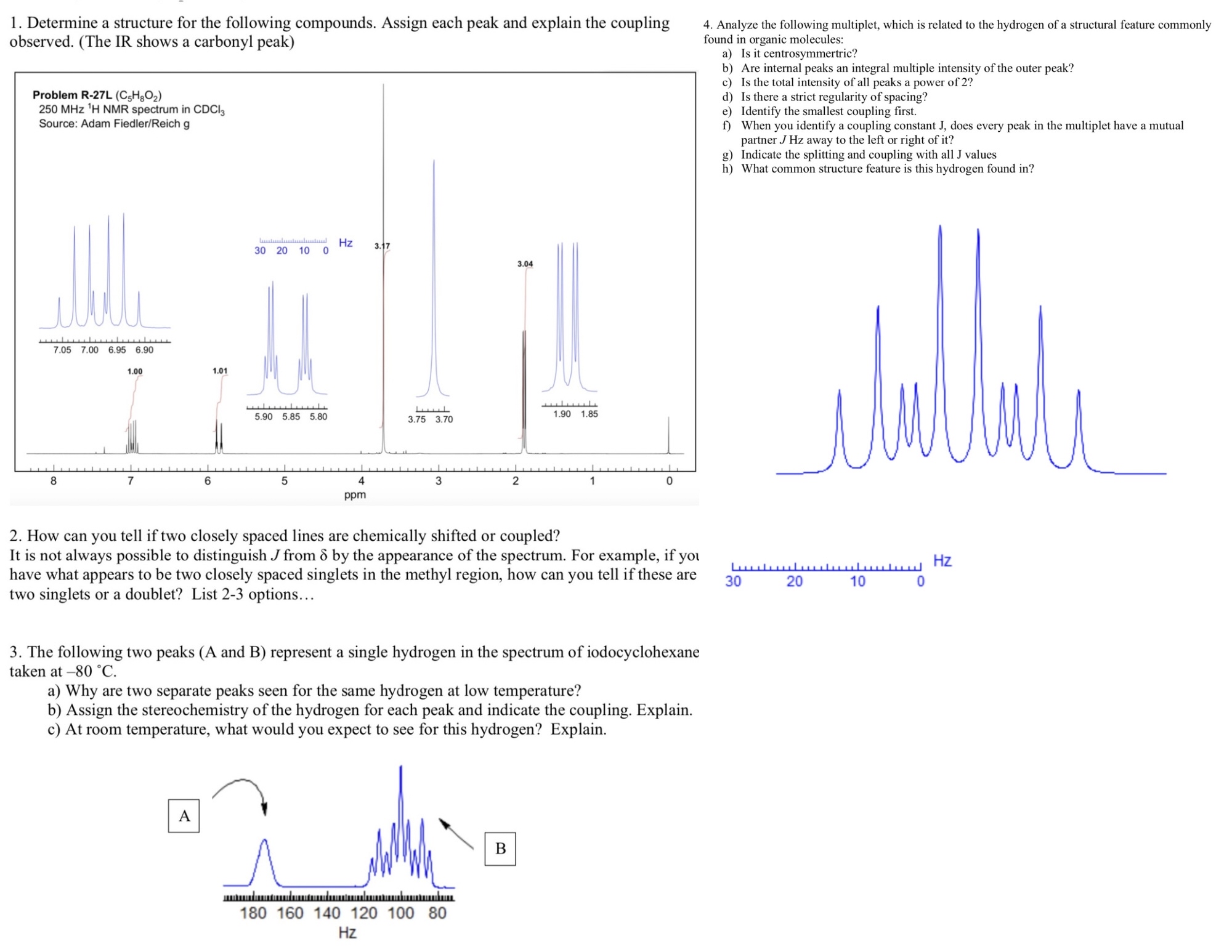
Analyze the following multiplet, which is related to the hydrogen of a structural feature commonly found in organic molecules:
a Is it centrosymmertric?
b Are internal peaks an integral multiple intensity of the outer peak?
c Is the total intensity of all peaks a power of
d Is there a strict regularity of spacing?
e Identify the smallest coupling first.
f When you identify a coupling constant does every peak in the multiplet have a mutual partner away to the left or right of it
g Indicate the splitting and coupling with all values
h What common structure feature is this hydrogen found in
The following two peaks A and B represent a single hydrogen in the spectrum of iodocyclohexane taken at
a Why are two separate peaks seen for the same hydrogen at low temperature?
b Assign the stereochemistry of the hydrogen for each peak and indicate the coupling. Explain.
c At room temperature, what would you
expect to see for this hydrogen? Explain.
How can you tell if two closely spaced lines are chemically shifted or coupled? It is not always possible to distinguish by the apperance of the spectrum.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


