Question: Help identify the compound! Draw and name it! also help identify IR spectrum peaks and assign the peaks given on the mass spectrum I was



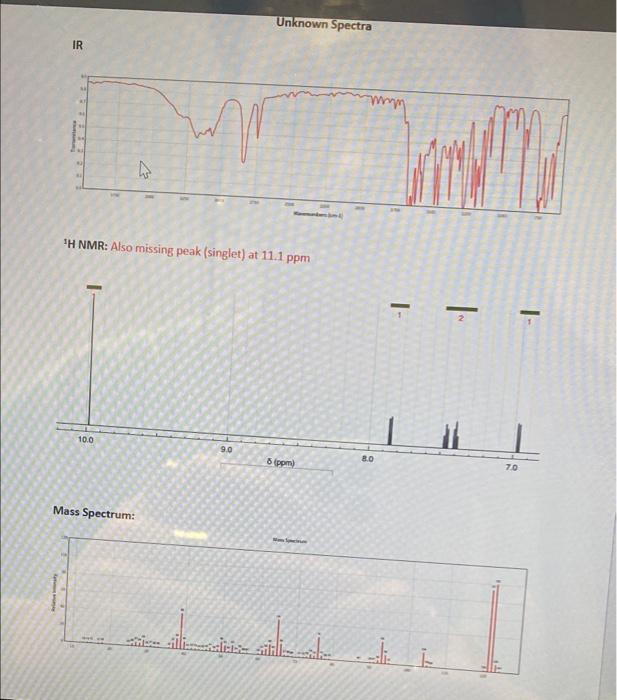
Qualitative Analysis Part II: Virtual Use the following information to determine the identity of an unknown compound. 1. Boiling Point 2. Chemical Tests 3. Melting Points of Derivative 4. Infrared Spectrum 5. Mass Spectrum 6. 'H NMR spectrum Possible identities are listed in tables in Canvas. The compound will contain either a ketone, aldehyde or alcohol functional group. Additional functional groups are possible, but it contains at least one of the three mentioned. 1. Boiling Point: The boiling point is determined to be approximately 163-165C using the set up below with the test tube heated in a sand bath. Note that this method sometimes leads to inaccurate readings because the boiling liquid often does not completely immerse the thermometer bulb. Liquid 2. The following chemical tests are run on the substance to determine functional groups in the molecule. Background information can be found on Canvas. The tests are not perfect and can give false results. Hypothetical results for the unknown for each test are given in italics. a. Bromine Unsaturation Test The bromine unsaturation test is a test for alkene/alkynes. Immediate disappearance of a the red/brown color of bromine is indication of a positive test. The test does not generally give positive tests for aromatic compounds or carbonyl groups. Reactions with these groups are typically much slower. Testing of the unknown compound does NOT result in rapid disappearance of the red/brown color of bromine. Styles b. Solubility Test Most standard organic compounds with standard functional groups have minimal reactivity with acid or base. As we saw in previous labs involving acid-base extractions, carboxylic acids will dissolve in aqueous 5% NaOH and aqueous 5% NaHCO3. Phenols dissolve in 5% NaOH, but not 5% NaHCO3. The unknown compound dissolved in both 5% NaOH and 5% NaHCO- c. Lucas Test This is a test for alcohols (but not for phenols). For a positive test, the -OH group is converted into a -Cl group which results in a product layer that is insoluble in the aqueous solution. The formation of the layer is fast with tertiary alcohols and slow with secondary alcohols. Primary alcohols typically show no reactivity. Phenols are also generally unreactive in the Lucas test. The test is also limited to alcohols that are soluble in the Lucas reagent (ZnCl, and HCl solution). Typically that means it generally is only useful for alcohols with 6 carbons or less or for alcohols with multiple -OH groups or other polar groups. The unknown compound dissolved, but did not produce a second layer. d. DNP Test The 2,4-dinitrophenylhydrazine (DNP) test typically produces an orange or red precipitate for aldehydes and ketones. The precipitate can be collected and its melting point taken. (Section 3). The unknown produces an orange precipitate in reaction with DNP reagent. e. Todoform Test The iodoform test produces a yellow precipitate (iodoform) with methyl ketones and secondary methyl alcohols from reaction with iodine and NaOH. The unknown did not a yellow precipitate in reaction with iodine and NaOH. f Chromicvnd Test The chromic acid test is positive for aldehydes, primary alcohols, and secondary alcohols through oxidation. The reaction typically produces a green solution or precipitate from formation of Cr* from the original orange solution of chromic acid with and oxidation state for chromium of Cro The unknown produced a green solution in reaction with chromic acid. g. Tollens Test The Tollens test is positive for aldehydes. The reaction produces a silver mirror when aldehyde reacts with the Tollens reagent. The unknown does not provide any visual signs of reactivity with the Tollens reagent. 3. Melting Point of derivatives. Reactions with semicarbazine, DNP reagent and oxime produced solids, which after purification, resulted in the following melting point readings. Semicarbazone: 228-229 C DNP: 140-142 C Oxime: 80-82 C 4-6. IR, Mass spectrum and 'H NMR are provided on Canvas. In real life, spectra may have impurities. No significant amounts are present here. Tasks to complete for this assignment. 1. Draw the unknown compound based on the given information and the tables of possible unknowns. II. Construct a table comparing the "data" for the unknown boiling point and derivative melting points versus the data listed on the tables. III. For each functional group test, comment on why the result agrees or disagrees with theory IV. Using chemdraw, draw chemical reactions that occurred in the positive chemical tests and derivative reactions. They should be with your proposed component and not with generic abbreviations, like "R.". If a reaction wasn't supposed to happen, then a drawing is not needed for that reaction. V. Write out a step-by-step mechanism of the DNP reaction for the unknown compound. VI. Identify peaks in the IR Spectrum relating to C-C, C-C-H, C-O, C-O, or O-H where applicable. Either write on the spectrum or write out answers as a data table. VII. Assign peaks on the 'H NMR spectrum to the structure. Use the picture or assign hydrogens to the chemical shift values. Use resonance structures and words to distinguish the two doublets in the *H NMR spectrum IX. Assign the peaks in the mass spectrum to the structure at the following positions. a. 121 m/z b. 122 m/z c. 123 m/z VIII. Unknown Spectra IR mm www 'H NMR: Also missing peak (singlet) at 11.1 ppm - E 10.0 9.0 6 ppm) 8.0 70 Mass Spectrum
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


