Question: 1. (do not pay attention to the selected options) 2. Describe each highlighted bond in terms of the overlap of atomic orbitals. (If the highlighted
1. (do not pay attention to the selected options)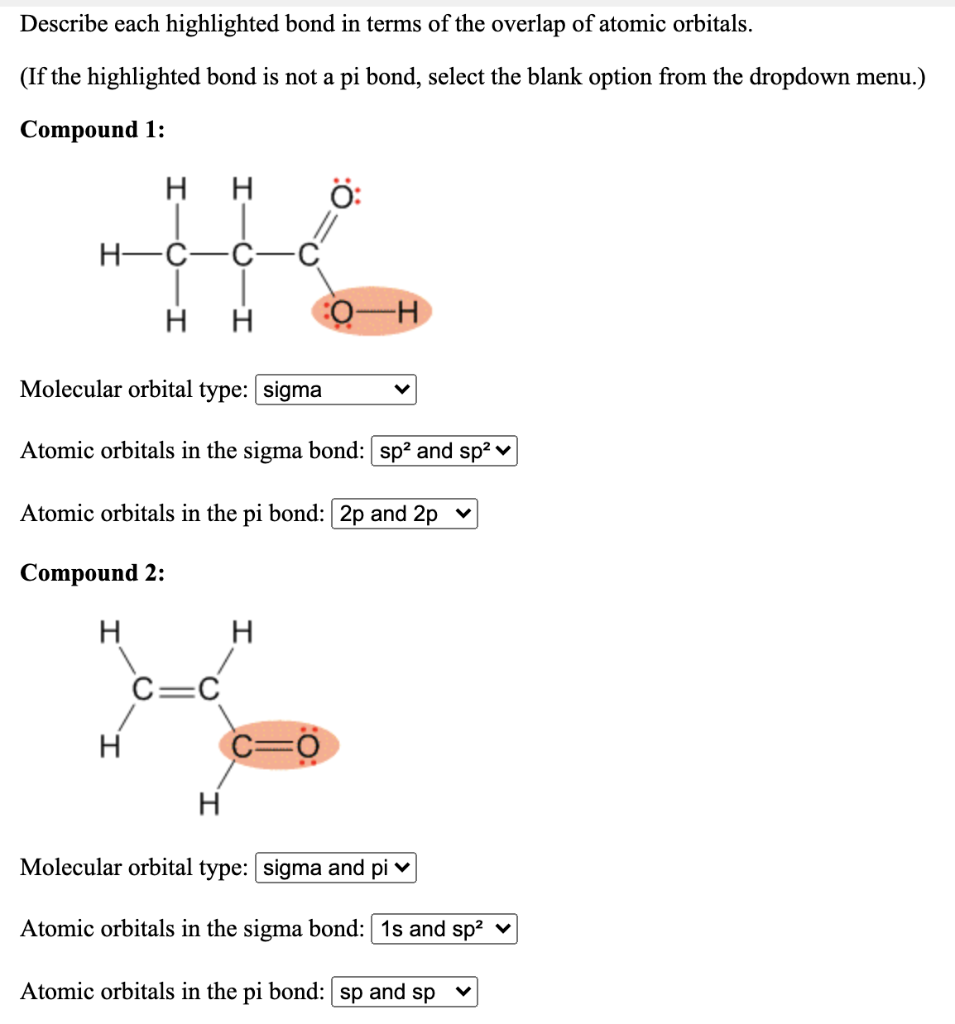
2.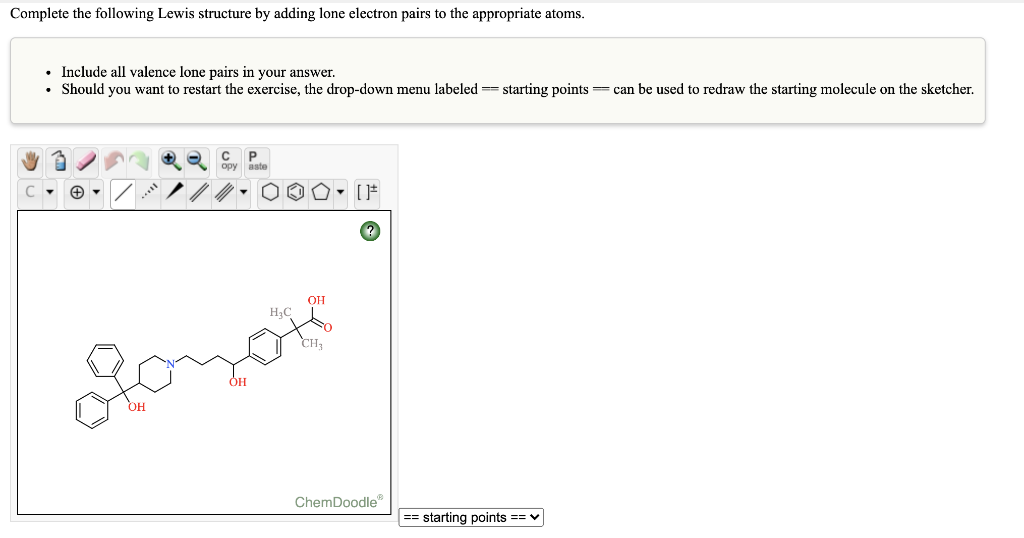
Describe each highlighted bond in terms of the overlap of atomic orbitals. (If the highlighted bond is not a pi bond, select the blank option from the dropdown menu.) Compound 1: . H : H-C . . - Molecular orbital type: sigma Atomic orbitals in the sigma bond: sp2 and spa v Atomic orbitals in the pi bond: 2p and 2p Compound 2: H H CEC . C=0 . Molecular orbital type: sigma and piv Atomic orbitals in the sigma bond: 1s and spa v Atomic orbitals in the pi bond: sp and sp Complete the following Lewis structure by adding lone electron pairs to the appropriate atoms. Include all valence lone pairs in your answer. Should you want to restart the exercise, the drop-down menu labeled == starting points=can be used to redraw the starting molecule on the sketcher. , aste [] otomoto ChemDoodle == starting points ==
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


