Question: 1. Draw Lewis dot structures for these molecules: CH4 NH3 H20 2. How many pairs of electrons are located around the central atom of each
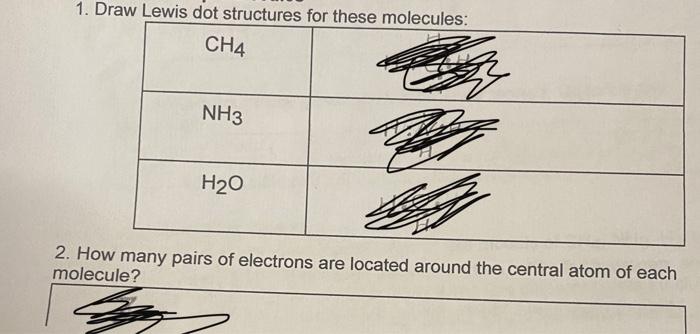

1. Draw Lewis dot structures for these molecules: CH4 NH3 H20 2. How many pairs of electrons are located around the central atom of each molecule? 3. Besides the identity of the central atom, what is different about these three molecules
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


