Question: 1. Draw Lewis structures for each compound: (A) SiCl (c) PH, S+3=H8 (d) SCr. (c) HIO, (f) H2CO; 2. Assign formal charges to each atom
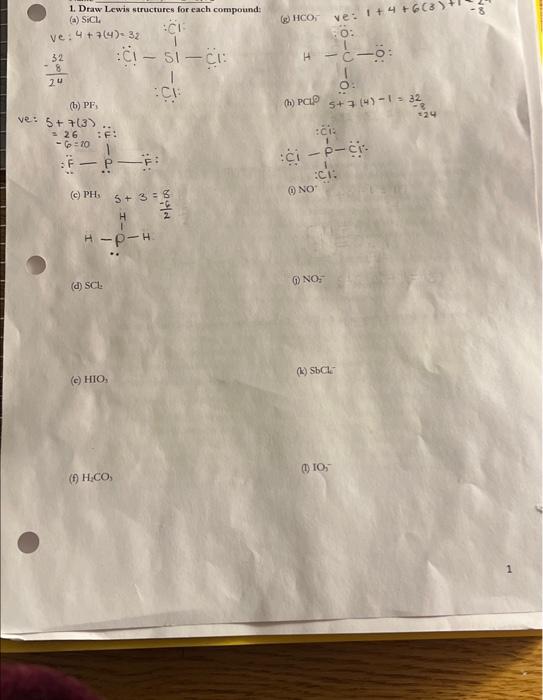
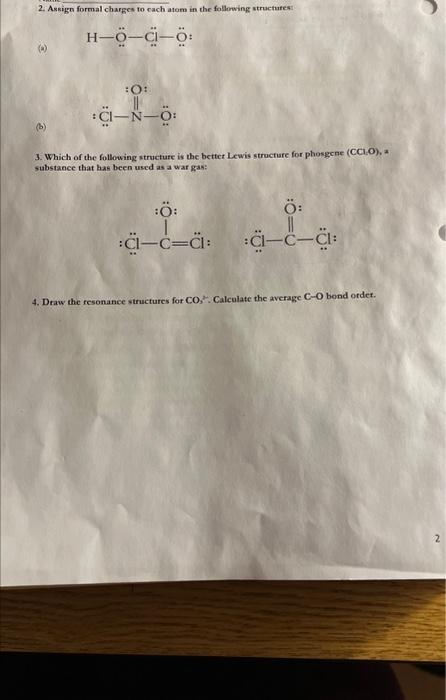
1. Draw Lewis structures for each compound: (A) SiCl (c) PH, S+3=H8 (d) SCr. (c) HIO, (f) H2CO; 2. Assign formal charges to each atom in the following structurest (a) (b) 3. Which of the following strueture is the better Lewis structure for phosgene (CCl,O), a substance that has been used as a war gas: 4. Draw the resonance structures for CO2. Calculate the average CO bond ordex
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


