Question: 1. Explain the difference between intermolecular forces and intramolecular forces. 2. List the six phases changes. Identify the exothermic changes and the endothermic ones. 3.
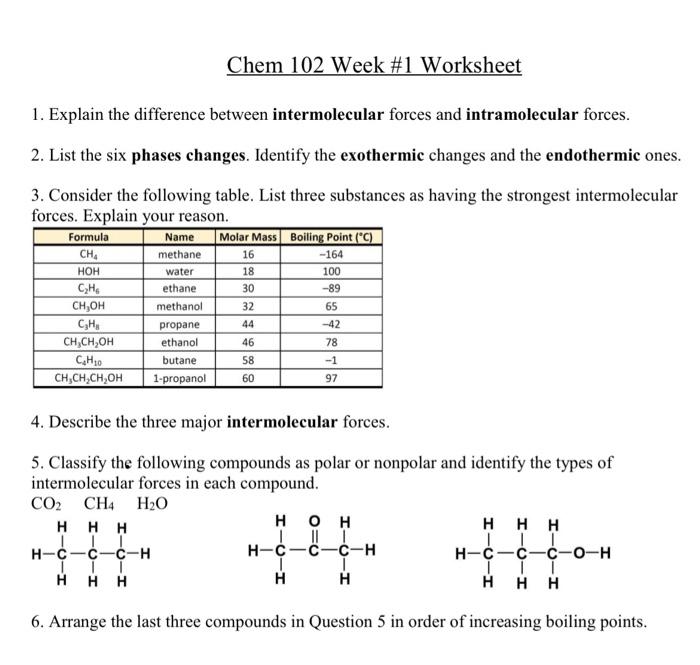
1. Explain the difference between intermolecular forces and intramolecular forces. 2. List the six phases changes. Identify the exothermic changes and the endothermic ones. 3. Consider the following table. List three substances as having the strongest intermolecular forces. Explain your reason. 4. Describe the three major intermolecular forces. 5. Classify the following compounds as polar or nonpolar and identify the types of intermolecular forces in each compound. 6. Arrange the last three compounds in Question 5 in order of increasing boiling points
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


