Question: 1. Fast Versus Slow Reactions It is common to add groups to difunctional molecules in two consecutive steps: ky A+B=C k_1 k2 C+B =D k-2
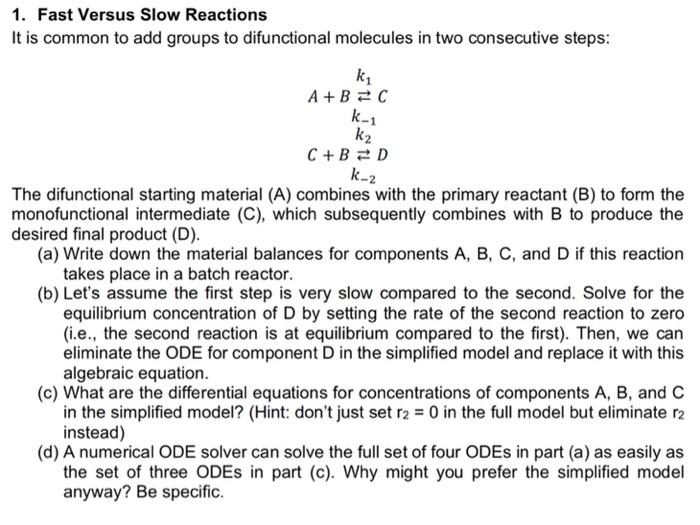
1. Fast Versus Slow Reactions It is common to add groups to difunctional molecules in two consecutive steps: ky A+B=C k_1 k2 C+B =D k-2 The difunctional starting material (A) combines with the primary reactant (B) to form the monofunctional intermediate (C), which subsequently combines with B to produce the desired final product (D). (a) Write down the material balances for components A, B, C, and D if this reaction takes place in a batch reactor. (b) Let's assume the first step is very slow compared to the second. Solve for the equilibrium concentration of D by setting the rate of the second reaction to zero (i.e., the second reaction is at equilibrium compared to the first). Then, we can eliminate the ODE for component D in the simplified model and replace it with this algebraic equation. (c) What are the differential equations for concentrations of components A, B, and C in the simplified model? (Hint: don't just set r2 = 0 in the full model but eliminate r2 instead) (d) A numerical ODE solver can solve the full set of four ODEs in part (a) as easily as the set of three ODEs in part (c). Why might you prefer the simplified model anyway? Be specific
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


