Question: Here is your personal data for this week's Dry Lab 5 - Carbohydrates: Carbohydrate unknown number - 3 Unknown sucrose solution number - 3 Tollen's





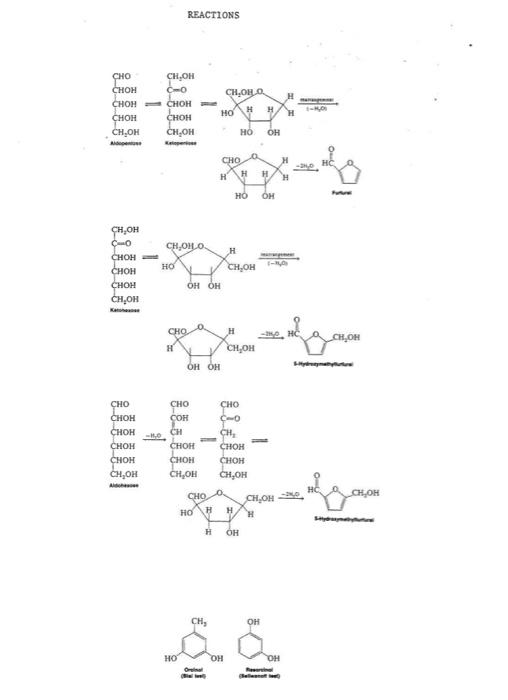

Here is your personal data for this week's Dry Lab 5 - Carbohydrates: Carbohydrate unknown number - 3 Unknown sucrose solution number - 3 Tollen's test - Silver mirror Barfoed's test - no red ppt Seliwanoff's test - no dark red Bial's test - brown solution Benedict's test - red ppt Observed rotation of sucrose solution - +37.9 degrees The description of the lab in Assignments lists the five possible carbohydrates. Read in your textbook about them and what categories they are in, such as hexose vs pentose, aldo vs keto, reducing vs nonreducing. Use the test result data that I emailed to you on Tuesday to determine what categories are true or not true about your unknown carbohydrate, using the test descriptions in the Instructions A. You can assume that fast reactions happen within 1 minute, and slow reactions happen in 5-10 minutes, and very slow reactions will not be observed to happen within the testing time. You should be able to eliminate some of the five possible carbohydrates and zero in on the one yours is. Carbohydrates Bial's Test for Pentoses: Place 2 mL of a 1% carbohydrate solution in a test tube. Piace 2 ml of distilled water into another test tube to serve as a control. Add 3 ml of Bial's reagent to each test tube. Carefully beat the tube above a Bunsen burner until the mixture just begins to boil. Note and record the color change. (If the color change is not obvious, add 5 mL of water and 1 ml of 1-pentanol to each test tube. After shaking, the l-pentanol layer should be colored.) Results and Conclusions: Pentoses yield blue-green condensation products while hexoses yield green, brown, or reddish-brown products. Seliwanoff's Test for Ketohexoses: Place 1 ml of a carbohydrate solution in a test tube. Place 1 ml of distilled water into another tube to serve as a control. Add 4 ml. of Seliwanoff's reagent to each tube. Place the tubes in a beaker of boiling water for 60 seconds. Remove the tubes and note the color change. Place the tubes back into the bath and, leaving the tubes in the bath, note the color change (1f any) at 1-ainute intervals for 5 minutes beyond the original 60-second heating period. Results and Conclusions: A ketohexose reacts rapidly to produce a dark red condensation product. An aidohexose reacts more slowly. Sucrose slowly hydrolyzes to yield fructose, which will react to give the dark red condensation product. Benedict's Test for Reducing Sugars: Place 1 ml of a 1% carbohydrate solution in a test tube. Place 1 ml of distilled water in a test tube to serve as a control. Add 5 mL of Benedict's reagent to each test tube. Place the tubes in a boiling-water bath for 2-3 minutes. Note the color changes. Results and Conclusions: A reducing sugar will produce a red, brown, or yellow precipitate. Reactions Bial's Test: furfural + orcinol + Feciz blue-green condensation product 5-hydroxymethylfurfural + orcinol + Feci, green, brown, reddish- brown condensation product Seliwanoff's Test: fast ketohexose 5-hydroxymethylfurfural resorcinol red product aldohexose slow resorcinol 5-hydroxymethylfurfural red product Benedict's Test: RCHO + 2cu2+ + 40+ RCOO + Cuzo + 2H20 Tollens' Test: RCHO + Ag (NH,) OR RCO0 + Ag Barfoed's Test: RCHO + 2cu2+ 2+ + 22,0 RCOOH + Cu O + 48* (Reducing monosaccharide: fast, reducing disaccharide: slow) Tollens' Test for Reducing Sugars: Mix 1 mL of Tollens' Reagent A with 1 l of Tollens' Reagent B in a small. beaker or Erlenmeyer flask. Add dilute (107) amonia solution until the black precipitate of silver oxide just dissolves. (Avoid adding an excess of ammonia solution.) Add 1 ml of the 12 carbohydrate solution into a clean test tube, add approximately 2-3 mL of the Tollens' reagent, and place the test tube in a warm water bath for several minutes. Note the development of the silver mirror. Results and Conclusion: A silver mirror indicates the presence of a reducing sugar--an aldose or ketose that can exist as a hemiacetal and that mutarotates. Barfoed's Test for Reducing Monosaccharides: Place 1 mL of a 12 carbohydrate solution in a test tube. Place 1 ml of distilled water in a test tube to serve as a control. Add 5 mL of Barfoed's reagent to each tube. Place the tubes in a boiling-water bath for 10 minutes. Note the presence of the red precipitate. Results and Conclusion: A red precipitate indicates the presence of a monosaccharide. Non- reducing disaccharides, such as sucrose, hydrolyze very slowly. Polarimetry: Determine the optical rotation for the known concentration of sucrose. Obtain a sample of sucrose of unknown concentration and determine its optical rotation. REACTIONS CHOLLO CHO , CHOH CHOH CHOM CH,OH CH,OH H H H HO CHO HC H H H HO CH,OHO CH.CH CH,OH H CH,OH CH, OH CHO H H CH, OH CHO CHO CHOH CHOH CH,OH , CHO CH CHOH CH,OH HOH CHOH CH,OH CHO CH OH OH CH, Carbohydrates Carbohydrate unknown number: IDENTITY OF CARBOHYDRATE Is your carbohydrate a pentose or a hexose? If your carbohydrate is a hexose, is it an aldohexose or a ketohexose? (Write "pentose" in the blank if your unknown is a pentose.) Does your carbohydrate give a positive Benedict's and/or Tollens' test? (yes or no) Is your carbohydrate a monosaccharide? (yes or no) Unknown sucrose solution number: What is the observed rotation (in degrees) of the unknown? What is the value of "c" (in grams of sucrose per milliliter of solution) of your unknown? Show all work below for this calculation. [a] = where a = observed rotation in degrees; c = concentration in grams per milliliter of solution; 1 = length of sample tube in decimeters; 1 = wavelength of light (usually indicated as "D," for the sodium D line); 1 = temperature in degrees Celsius
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


